Pan-cancer analysis of MET mutation and its association with the efficacy of immune checkpoint blockade
- PMID: 40330151
- PMCID: PMC12053711
- DOI: 10.1016/j.gendis.2024.101450
Pan-cancer analysis of MET mutation and its association with the efficacy of immune checkpoint blockade
Erratum in
-
Corrigendum to "Pan-cancer analysis of MET mutation and its association with the efficacy of immune checkpoint blockade" [Genes & Dis 12 (2025) 101450].Genes Dis. 2025 Jun 13;12(6):101726. doi: 10.1016/j.gendis.2025.101726. eCollection 2025 Nov. Genes Dis. 2025. PMID: 40837412 Free PMC article.
Abstract
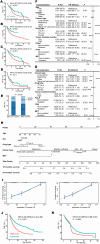
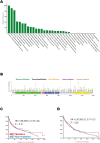
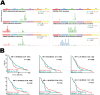
The mesenchymal-epithelial transition factor (MET) proto-oncogene plays important roles during tumor development. Recently, evidence has revealed MET signaling may impact tumor immunogenicity and regulate the immune response. Here we conducted a comprehensive bioinformatic and clinical analysis to explore the characteristics of MET mutation and its association with the outcomes in pan-cancer immunotherapy. In 4149 patients with 12 tumor types treated with immune checkpoint inhibitors, MET mutation indicated favorable overall survival (hazard ratio = 0.61; 95% CI, 0.50-0.74; P < 0.001), progression-free survival (hazard ratio = 0.74; 95% CI, 0.60-0.92; P = 0.01), and objective response rate (40.3% vs. 28.1%; P = 0.003). Moreover, we developed a nomogram to estimate the 12-month and 24-month survival probabilities after the initiation of immunotherapy. Further multi-omics analysis on both intrinsic and extrinsic immune landscapes revealed that MET mutation enhanced tumor immunogenicity, enriched infiltration of immune cells, and improved immune responses. In summary, MET mutation improves cancer immunity and is an independent biomarker for favorable outcomes in pan-cancer immunotherapy. These results may influence clinical practices, guide treatment decision-making, and develop immunotherapy for personalized care.
Keywords: Biomarker; Cancer; Immune checkpoint inhibitor; Immunotherapy; Mesenchymal-epithelial transition factor; Tumor immunogenicity.
© 2024 The Authors. Publishing services by Elsevier B.V. on behalf of KeAi Communications Co., Ltdé.
Conflict of interest statement
All authors claimed no competing interests.
Figures


Similar articles
-
Interplay between tumor mutation burden and the tumor microenvironment predicts the prognosis of pan-cancer anti-PD-1/PD-L1 therapy.Front Immunol. 2025 Jul 24;16:1557461. doi: 10.3389/fimmu.2025.1557461. eCollection 2025. Front Immunol. 2025. PMID: 40777041 Free PMC article.
-
Systemic treatments for metastatic cutaneous melanoma.Cochrane Database Syst Rev. 2018 Feb 6;2(2):CD011123. doi: 10.1002/14651858.CD011123.pub2. Cochrane Database Syst Rev. 2018. PMID: 29405038 Free PMC article.
-
Mutation of lysine-specific demethylase 5 is associated with enhanced tumor immunity and favorable outcomes in pan-cancer immune checkpoint blockade.Mol Cancer. 2024 Dec 27;23(1):281. doi: 10.1186/s12943-024-02197-3. Mol Cancer. 2024. PMID: 39731135 Free PMC article.
-
Comprehensive pan-cancer analysis reveals NTN1 as an immune infiltrate risk factor and its potential prognostic value in SKCM.Sci Rep. 2025 Jan 25;15(1):3223. doi: 10.1038/s41598-025-85444-x. Sci Rep. 2025. PMID: 39863609 Free PMC article.
-
Immune checkpoint inhibitors plus platinum-based chemotherapy compared to platinum-based chemotherapy with or without bevacizumab for first-line treatment of older people with advanced non-small cell lung cancer.Cochrane Database Syst Rev. 2024 Aug 13;8(8):CD015495. doi: 10.1002/14651858.CD015495. Cochrane Database Syst Rev. 2024. PMID: 39136258 Free PMC article.
References
-
- Hegde P.S., Chen D.S. Top 10 challenges in cancer immunotherapy. Immunity. 2020;52(1):17–35. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

