Specific effects on liver relevant for performing a dietary cumulative risk assessment of pesticide residues
- PMID: 40330216
- PMCID: PMC12050957
- DOI: 10.2903/j.efsa.2025.9409
Specific effects on liver relevant for performing a dietary cumulative risk assessment of pesticide residues
Abstract
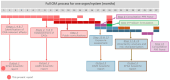
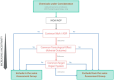
According to the 'EFSA-SANTE Action Plan on Cumulative Risk Assessment for pesticides residues', EFSA initiated a retrospective cumulative risk assessment (CRA) of the effects of pesticide residues on the liver. For this CRA, EFSA identified the following liver-specific effects in accordance with the International Harmonisation of Nomenclature and Diagnostic Criteria (INHAND): (1) hypertrophy due to enzymatic induction, liver; (2) fatty change and/or phospholipidosis, hepatocellular; (3) degeneration/cell death, hepatocellular; (4) porphyria, hepatocellular, biliary duct; (5) cholestasis, hepatocellular, biliary duct; (6) preneoplastic and neoplastic changes, hepatocellular; (7) neoplastic changes, biliary duct. In addition, as gallbladder is part of the extrahepatic biliary system and can be affected by hepatic toxicity, the following specific effects in the gallbladder were defined: (1) erosion/ulceration, gallbladder (2) calculi, gallbladder and (3) neoplastic changes, gallbladder. Histopathology was considered as the most appropriate source of evidence together with the increase in relative liver weight, and a list of indicators was defined and will be used to collect information on these specific effects as included in the assessment reports of the different active substances used as plant protection products. The criteria for inclusion of active substances/metabolites into cumulative assessment groups (CAGs) were also defined, together with the hazard characterisation methodology and the lines of evidence for assessing CAG-membership probabilities. While primary indicators define the specific effect, secondary indicators and other endpoints (named ancillary endpoints) are considered not sufficiently informative to indicate a specific effect but are rather contributing to the overall evidence; these will be collected only for a limited number of substances (i.e. risk drivers based on hazard and exposure considerations) for determining the likelihood of the substances truly belonging to the CAGs (CAG-membership probabilities). Considering that it is not considered appropriate to establish CAGs for acute liver effects, CRAs on the liver will be only focused on chronic exposure. The process of data extraction and actual establishment of the CAGs is beyond the scope of this report. This part of the CRA process was outsourced and will be the subject of a separate report.
Keywords: cumulative assessment groups; cumulative risk assessment; gallbladder; liver; pesticide residues; specific effects.
© 2025 European Food Safety Authority. EFSA Journal published by Wiley‐VCH GmbH on behalf of European Food Safety Authority.
Figures

Similar articles
-
Specific effects on kidneys relevant for performing a dietary cumulative risk assessment of pesticide residues.EFSA J. 2025 May 5;23(5):e9406. doi: 10.2903/j.efsa.2025.9406. eCollection 2025 May. EFSA J. 2025. PMID: 40330214 Free PMC article.
-
Specific effects on the thyroid relevant for performing a dietary cumulative risk assessment of pesticide residues: 2024 update.EFSA J. 2024 Mar 18;22(3):e8672. doi: 10.2903/j.efsa.2024.8672. eCollection 2024 Mar. EFSA J. 2024. PMID: 38500786 Free PMC article.
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Dietary glycation compounds - implications for human health.Crit Rev Toxicol. 2024 Sep;54(8):485-617. doi: 10.1080/10408444.2024.2362985. Epub 2024 Aug 16. Crit Rev Toxicol. 2024. PMID: 39150724
-
Critical appraisal of the expert knowledge elicitation (EKE) methodology to identify uncertainties in building cumulative assessment groups for craniofacial alterations.Reprod Toxicol. 2025 Mar;132:108753. doi: 10.1016/j.reprotox.2024.108753. Epub 2024 Nov 22. Reprod Toxicol. 2025. PMID: 39580099
References
-
- Boone, L. , Meyer, D. , Cusick, P. , Ennulat, D. , Bolliger, A. P. , Everds, N. , Meador, V. , Elliott, G. , Honor, D. , Bounous, D. , & Jordan, H. (2005). Selection and interpretation of clinical pathology indicators of hepatic injury in preclinical studies. Veterinary Clinical Pathology, 34(3), 182–188. 10.1111/j.1939-165X.2005.tb00041.x - DOI - PubMed
-
- Cattley, R. C. , & Cullen, J. M. (2018). Liver and Gall Bladder. In Wallig M. A., Haschek W. M., Rousseaux C., & Bolon B. (Eds.), Fundamentals of Toxicologic pathology (3rd ed., pp. 125–151). Academic Press. 10.1016/B978-0-12-809841-7.00008-3 - DOI
-
- Chang, Z. , Guan, J.‐X. , Hui, D.‐Y. , Zhang, N.‐N. , Lu, L.‐R. , Tang, L.‐Y. , Shao, C.‐K. , & Chen, J.‐N. (2022). Liver involvement in patients with erythropoietic protoporphyria: Retrospective analysis of clinicopathological features of 5 cases. Annals of Diagnostic Pathology, 56, 151859. 10.1016/j.anndiagpath.2021.151859 - DOI - PubMed
-
- Crawford, J. M. , & Liu, C. (2021). Chapter 18: Liver and Gallblader. In Kumar V., Abbas A. K., & Aster J. C. (Eds.), Robbins and Cotran pathologic basis of disease (10th ed., pp. 821–882). Elsevier.
LinkOut - more resources
Full Text Sources
