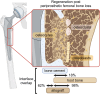
Allografts promote skeletal regeneration of periprosthetic femoral bone loss
- PMID: 40330274
- PMCID: PMC12053977
- DOI: 10.1016/j.jot.2025.04.004
Allografts promote skeletal regeneration of periprosthetic femoral bone loss
Abstract
Background: Periprosthetic bone loss is a common clinical problem in hip arthroplasty that must be addressed during revision surgery to achieve adequate implant stability. Although bone allografts represent the clinical standard among substitute materials used, evidence of their regenerative potential at the microstructural, cellular, and compositional level is lacking.
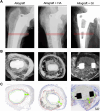
Methods: A multiscale imaging approach comprising contact radiography, undecalcified histology, scanning electron microscopy, and nanoindentation was employed on human femoral explants obtained postmortem many years after allograft use during revision surgery.
Results: The degree of skeletal regeneration through allograft incorporation between host bone and allograft bone was highly dependent on the defect depth (R2 = 0.94, p < 0.001), while no association between the allograft time in situ and incorporation (R2 = 0.06, p = 0.61) was apparent. The host bone-allograft interface showed a high overlap of 4.0 ± 2.9 mm and was characterized by active bone remodelling, as indicated by osteoid accumulation, high abundance of bone cells and vasculature. While bone cement generally limited the incorporation process, the osteocytic canalicular system of the host bone reached the allograft interface to guide bone remodelling.
Conclusion: This is the first multiscale, histomorphometry-based evaluation of bone allografts used in revision hip arthroplasty for femoral bone loss in humans, demonstrating that they adequately facilitate skeletal regeneration through osteoconduction and subsequent remodelling.
The translational potential of this article: This study identified the mechanisms and determinants of femoral defect regeneration through allografts on the basis of a unique sample collection. While our results support their favourable clinical outcomes, the scientific basis for incomplete incorporation is also demonstrated.
Keywords: Allograft; Bone regeneration; Bone transplantation; Osseointegration; Osteocyte; Revision arthroplasty.
© 2025 The Authors.
Conflict of interest statement
All authors declare that there are no conflicts of interest in relation to this work.
Figures





Similar articles
-
Incorporation and Remodeling of Structural Allografts in Acetabular Reconstruction: Multiscale, Micro-Morphological Analysis of 13 Pelvic Explants.J Bone Joint Surg Am. 2018 Aug 15;100(16):1406-1415. doi: 10.2106/JBJS.17.01636. J Bone Joint Surg Am. 2018. PMID: 30106822 Free PMC article.
-
Allograft Chip Incorporation in Acetabular Reconstruction: Multiscale Characterization Revealing Osteoconductive Capacity.J Bone Joint Surg Am. 2021 Nov 3;103(21):1996-2005. doi: 10.2106/JBJS.20.01943. J Bone Joint Surg Am. 2021. PMID: 34228665
-
A novel, multi-level approach to assess allograft incorporation in revision total hip arthroplasty.Sci Rep. 2020 Sep 16;10(1):15226. doi: 10.1038/s41598-020-72257-3. Sci Rep. 2020. PMID: 32939007 Free PMC article.
-
Femoral stem impaction grafting: extending the role of cement.Bone Joint J. 2013 Nov;95-B(11 Suppl A):92-4. doi: 10.1302/0301-620X.95B11.32762. Bone Joint J. 2013. PMID: 24187362 Review.
-
Grafting for periprosthetic femoral fractures: strut, impaction or femoral replacement.Injury. 2007 Jun;38(6):688-97. doi: 10.1016/j.injury.2007.02.046. Epub 2007 Apr 27. Injury. 2007. PMID: 17466991 Review.
Cited by
-
"Multidisciplinary synergy driving innovation in orthopaedic translational medicine".J Orthop Translat. 2025 Jun 4;52:A1-A3. doi: 10.1016/j.jot.2025.06.001. eCollection 2025 May. J Orthop Translat. 2025. PMID: 40698066 Free PMC article. No abstract available.
References
-
- Learmonth I.D., Young C., Rorabeck C. The operation of the century: total hip replacement. Lancet. 2007;370(9597):1508–1519. - PubMed
-
- Pivec R., Johnson A.J., Mears S.C., Mont M.A. Hip arthroplasty. Lancet. 2012;380(9855):1768–1777. - PubMed
-
- Mayle R.E., Jr., Paprosky W.G. Massive bone loss: allograft-prosthetic composites and beyond. J Bone Joint Surg Br. 2012;94-B:61–64. - PubMed
-
- Uchiyama K., Inoue G., Takahira N., Takaso M. Revision total hip arthroplasty - salvage procedures using bone allografts in Japan. J Orthop Sci. 2017;22(4):593–600. - PubMed
-
- Sheth N.P., Nelson C.L., Springer B.D., Fehring T.K., Paprosky W.G. Acetabular bone loss in revision total hip arthroplasty: evaluation and management. J Am Acad Orthop Surg. 2013;21(3):128–139. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

