Impact of Culture Duration on the Properties and Functionality of Yeast-Derived Extracellular Vesicles
- PMID: 40330275
- PMCID: PMC12053258
- DOI: 10.34133/bmr.0201
Impact of Culture Duration on the Properties and Functionality of Yeast-Derived Extracellular Vesicles
Abstract
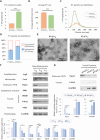
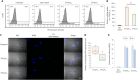
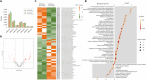
Extracellular vesicles (EVs), lipid bilayer nanovesicles secreted by cells, carry nucleic acids, proteins, and other bioactive molecules that influence recipient cells and modulate various biological processes. This study investigated how energy depletion and fermentation processes influence the characteristics and physiological functions of EVs secreted by Saccharomyces cerevisiae. Specifically, we analyzed EVs derived from 24-h cultures, representing the glucose utilization phase, and 72-h cultures, representing the starvation stage. Under energy-depleted conditions (72-h cultures), yeast secreted a higher number of EV particles, albeit with a smaller average particle size. In contrast, EVs from yeast cultured for 24 h, during the glucose utilization phase, were enriched in Pep12-rich endosome-derived vesicles and exhibited 71% higher cellular internalization efficiency. Proteomic and transcriptomic analyses revealed distinct protein and microRNA profiles between EVs from 24- and 72-h cultures, highlighting their potential roles in tissue regeneration, cell proliferation, and collagen synthesis. As a result, EVs derived from 24-h cultures exhibited a 15% greater effect in promoting collagen synthesis. The differential effects on collagen production may be attributed to the efficiency of endocytosis and the specific protein and microRNA cargo of the EVs. This study emphasizes the functional potential and unique properties of yeast-derived EVs while proposing strategies to modulate EV composition by adjusting the yeast culture duration and the energy source in the medium. Further research is needed to control yeast-produced EV components and to understand their mechanisms of action for effective therapeutic applications.
Copyright © 2025 Gyeongchan Jeon et al.
Conflict of interest statement
Competing interests: The authors declare that they have no competing interests.
Figures



Similar articles
-
Environmental conditions modulate the protein content and immunomodulatory activity of extracellular vesicles produced by the probiotic Propionibacterium freudenreichii.Appl Environ Microbiol. 2021 Mar 1;87(4):e02263-20. doi: 10.1128/AEM.02263-20. Epub 2020 Dec 11. Appl Environ Microbiol. 2021. PMID: 33310709 Free PMC article.
-
Endometrial extracellular vesicles regulate processes related to embryo development and implantation in human blastocysts.Hum Reprod. 2025 Jan 1;40(1):56-68. doi: 10.1093/humrep/deae256. Hum Reprod. 2025. PMID: 39576620
-
Scalable Production of Extracellular Vesicles and Its Therapeutic Values: A Review.Int J Mol Sci. 2022 Jul 20;23(14):7986. doi: 10.3390/ijms23147986. Int J Mol Sci. 2022. PMID: 35887332 Free PMC article. Review.
-
Exploring Extracellular Vesicles of Probiotic Yeast as Carriers of Biologically Active Molecules Transferred to Human Intestinal Cells.Int J Mol Sci. 2023 Jul 12;24(14):11340. doi: 10.3390/ijms241411340. Int J Mol Sci. 2023. PMID: 37511103 Free PMC article.
-
An Emerging Frontier in Intercellular Communication: Extracellular Vesicles in Regeneration.Front Cell Dev Biol. 2022 May 11;10:849905. doi: 10.3389/fcell.2022.849905. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35646926 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials

