Multi-omics identification of a polyamine metabolism related signature for hepatocellular carcinoma and revealing tumor microenvironment characteristics
- PMID: 40330470
- PMCID: PMC12052762
- DOI: 10.3389/fimmu.2025.1570378
Multi-omics identification of a polyamine metabolism related signature for hepatocellular carcinoma and revealing tumor microenvironment characteristics
Abstract
Background: Accumulating evidence indicates that elevated polyamine levels are closely linked to tumor initiation and progression. However, the precise role of polyamine metabolism in hepatocellular carcinoma (HCC) remains poorly understood.
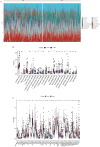
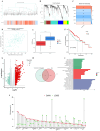
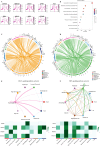
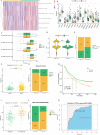
Methods: We conducted differential expression analysis on bulk RNA sequencing data from The Cancer Genome Atlas (TCGA) and Gene Expression Omnibus (GEO) to identify 65 polyamine metabolism-related genes. By employing unsupervised consensus clustering, AddModuleScore, single-sample gene set enrichment analysis (ssGSEA), and weighted gene co-expression network analysis (WGCNA), we identified polyamine metabolism-related genes at both the bulk RNA-seq and single-cell RNA-seq (scRNA-seq) levels. Utilizing 101 machine learning algorithms, we constructed a polyamine metabolism-related signature (PMRS) and validated its predictive power across training, testing, and external validation cohorts. Additionally, we developed a prognostic nomogram model by integrating PMRS with clinical variables. To explore immune treatment sensitivity, we assessed tumor mutation burden (TMB), tumor immune dysfunction and exclusion (TIDE) score, mutation frequency, and immune checkpoint genes expression. Immune cell infiltration was analyzed using the CIBERSORT algorithm. Finally, RT-qPCR experiments were conducted to validate the expression of key genes.
Results: Using 101 machine learning algorithms, we established a polyamine metabolism-related signature comprising 9 genes, which exhibited strong prognostic value for HCC patients. Further analysis revealed significant differences in clinical features, biological functions, mutation profiles, and immune cell infiltration between high-risk and low-risk groups. Notably, TIDE analysis and immune phenotype scoring (IPS) demonstrated distinct immune treatment sensitivities between the two risk groups. RT-qPCR validation confirmed that these 9 genes were highly expressed in normal cells but significantly downregulated in tumor cells.
Conclusions: Our study developed a polyamine metabolism-based prognostic risk signature for HCC, which may provide valuable insights for personalized treatment strategies in HCC patients.
Keywords: hepatocellular carcinoma; immune therapy; machine learning; multi-omics analysis; polyamine metabolism; single-cell RNA sequencing.
Copyright © 2025 Yu, Liu, Liu, Zhao, Zhang, Jiang and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures











Similar articles
-
Integrated single-cell and bulk transcriptome analysis of R-loop score-based signature with regard to immune microenvironment, lipid metabolism and prognosis in HCC.Front Immunol. 2025 Jan 9;15:1487372. doi: 10.3389/fimmu.2024.1487372. eCollection 2024. Front Immunol. 2025. PMID: 39850878 Free PMC article.
-
Integrated Analysis of Single-Cell and Bulk RNA-Sequencing Defines N7-Methylguanosine (m7G)-Mediated Modifications' Role in Prognosis and the Tumor Immune Microenvironment in Hepatocellular Carcinoma.Cancer Med. 2025 Jun;14(11):e70992. doi: 10.1002/cam4.70992. Cancer Med. 2025. PMID: 40485623 Free PMC article.
-
METTL1-driven nucleotide metabolism reprograms the immune microenvironment in hepatocellular carcinoma: a multi-omics approach for prognostic biomarker discovery.Front Immunol. 2025 Apr 22;16:1582203. doi: 10.3389/fimmu.2025.1582203. eCollection 2025. Front Immunol. 2025. PMID: 40330476 Free PMC article.
-
Polyamine metabolism and anti-tumor immunity.Front Immunol. 2025 Feb 18;16:1529337. doi: 10.3389/fimmu.2025.1529337. eCollection 2025. Front Immunol. 2025. PMID: 40040695 Free PMC article. Review.
-
Identification of drivers from cancer genome diversity in hepatocellular carcinoma.Int J Mol Sci. 2014 Jun 20;15(6):11142-60. doi: 10.3390/ijms150611142. Int J Mol Sci. 2014. PMID: 24955791 Free PMC article. Review.
Cited by
-
Metabolic reprogramming and prognostic insights in molecular landscapes driven by glycolysis in ovarian cancer.Sci Rep. 2025 Jul 24;15(1):26956. doi: 10.1038/s41598-025-12350-7. Sci Rep. 2025. PMID: 40707588 Free PMC article.
References
-
- Galle PR, Finn RS, Qin S, Ikeda M, Zhu AX, Kim TY, et al. . Patient-reported outcomes with atezolizumab plus bevacizumab versus sorafenib in patients with unresectable hepatocellular carcinoma (IMbrave150): an open-label, randomised, phase 3 trial. Lancet Oncol. (2021) 22:991–1001. doi: 10.1016/S1470-2045(21)00151-0 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

