Serum angiotensin type-1 receptor autoantibodies and neurofilament light chain as markers of neuroaxonal damage in post-COVID patients
- PMID: 40330487
- PMCID: PMC12052551
- DOI: 10.3389/fimmu.2025.1571027
Serum angiotensin type-1 receptor autoantibodies and neurofilament light chain as markers of neuroaxonal damage in post-COVID patients
Abstract
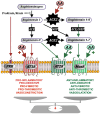
Introduction: Dysregulation of autoimmune responses and the presence of autoantibodies (AA), particularly those related to the renin-angiotensin system (RAS), have been implicated in the acute phase of COVID-19, and persistent dysregulation of brain RAS by RAS-related autoantibodies may also contribute to neurological symptoms of post-COVID.
Methods: We analyzed levels of serum and CSF RAS AA in post-COVID patients with neurological symptoms, individuals who have fully recovered from COVID-19 (after-COVID controls), and uninfected individuals, and their possible correlations with the serum marker of neuroaxonal damage neurofilament light chain (NfL) and the degrees of cognitive deficit.
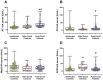
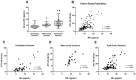
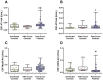
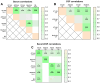
Results: Both in serum and CSF, levels of AA agonists of the pro-inflammatory angiotensin II type 1 receptors (AT1-AA) were significantly elevated in this cohort of neurological post-COVID patients compared to both uninfected and after-COVID controls and correlated with serum levels of NfL. Changes in serum and CSF levels of AA promoting the RAS anti-inflammatory axis (upregulation of AA agonists of AT2 and Mas receptors, downregulation of AA antagonists of ACE2) suggest upregulation of the RAS compensatory response in this cohort of neurological post-COVID patients. Post-COVID patients with more pronounced cognitive impairment exhibited significantly higher CSF levels of MasR-AA and a trend toward elevated AT2-AA. Persistent brain RAS dysregulation, particularly persistent increase in AT1-AA, and its correlation with neuroaxonal damage markers and cognitive impairment, may play a significant role in neurological symptoms associated with post-COVID. Serum levels of NfL and AT1-AA may be interesting biomarkers for the early identification of CNS involvement in patients with neurological symptoms and a history of COVID-19. However, post-COVID is a highly heterogeneous entity and may result from various underlying mechanisms. The present study includes a cohort, which may differ from other cohorts with different clinical profiles, which may show different results on NfLs and CSF RAS autoantibodies, particularly AT1-AA.
Conclusion: These findings highlight the potential of targeting AT1 receptors as a therapeutic strategy for mitigating cognitive deficits in post-COVID patients showing upregulated AT1-AA levels.
Keywords: AT1; COVID-19; autoantibody; autoimmunity; biomarkers; cognitive impairment; neurological long-COVID; post-acute sequelae of COVID-19 syndrome.
Copyright © 2025 Rodriguez-Perez, Serrano-Heras, Labandeira, Camacho-Meño, Castro-Robles, Suarez-Quintanilla, Muñoz-López, Piqueras-Landete, Guerra, Segura and Labandeira-Garcia.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures

References
-
- Diez-Cirarda M, Yus-Fuertes M, Sanchez-Sanchez R, Gonzalez-Rosa JJ, Gonzalez-Escamilla G, Gil-Martinez L, et al. Hippocampal subfield abnormalities and biomarkers of pathologic brain changes: from sars-cov-2 acute infection to post-covid syndrome. EBioMedicine. (2023) 94:104711. doi: 10.1016/j.ebiom.2023.104711 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

