Mutation in the Unrearranged PML Allele Confers Resistance to Arsenic Trioxide in Acute Promyelocytic Leukemia
- PMID: 40330660
- PMCID: PMC12053449
- DOI: 10.34133/research.0696
Mutation in the Unrearranged PML Allele Confers Resistance to Arsenic Trioxide in Acute Promyelocytic Leukemia
Abstract
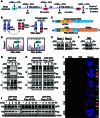
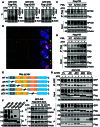
Arsenic trioxide (ATO) is able to selectively target and degrade the disease-causing PML::RARα (P/R) oncoprotein in acute promyelocytic leukemia (APL) for curing the disease. However, some relapsed patients develop resistance to ATO due to mutations in the promyelocytic leukemia (PML) part of the PML::RARα fusion gene. A relapsed APL patient had shown resistance to ATO and chemotherapy and was identified to harbor a point mutation (A216V) in the unrearranged PML allele rather than the PML::RARα fusion gene. Here, we report that mutations in the unrearranged PML allele impede the ATO-induced destabilization and degradation of the wild-type P/R oncoprotein. Deletion of the coiled-coil domain in a PML mutant completely reversed wild-type P/R protein resistance to ATO by abolishing the interaction between PML and P/R proteins. Collectively, our findings reveal that a point mutation in the unrearranged PML allele can confer ATO resistance through a protein-protein interaction. Therefore, the unrearranged PML allele should also be screened for drug-resistant mutations in relapsed APL patients.
Copyright © 2025 Pei-Han Yu et al.
Conflict of interest statement
Competing interests: The authors declare that they have no competing interests.
Figures
References
-
- Thé H, Chomienne C, Lanotte M, Degos L, Dejean A. The t(15;17) translocation of acute promyelocytic leukaemia fuses the retinoic acid receptor α gene to a novel transcribed locus. Nature. 1990;347(6293):558–561. - PubMed
-
- Grignani F, Ferrucci PF, Testa U, Talamo G, Fagioli M, Alcalay M, Mencarelli A, Grignani F, Peschle C, Nicoletti I, et al. The acute promyelocytic leukemia-specific PML-RARα fusion protein inhibits differentiation and promotes survival of myeloid precursor cells. Cell. 1993;74(3):423–431. - PubMed
-
- Zhang XW, Yan XJ, Zhou ZR, Yang FF, Wu ZY, Sun HB, Liang WX, Song AX, Lallemand-Breitenbach V, Jeanne M, et al. Arsenic trioxide controls the fate of the PML-RARα oncoprotein by directly binding PML. Science. 2010;328(5975):240–243. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

