Reproductive Cold Stress in Contrasting Sorghum Genotypes: Is Pollen Fertility Really the Crucial Trait?
- PMID: 40330702
- PMCID: PMC12050216
- DOI: 10.1002/pld3.70065
Reproductive Cold Stress in Contrasting Sorghum Genotypes: Is Pollen Fertility Really the Crucial Trait?
Abstract
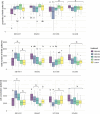
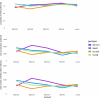
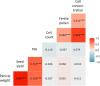
The influence of cold stress during the reproductive phase can lead to substantial yield losses in sorghum. In order to extend cultivation into temperate regions, a better understanding of reproductive cold tolerance is essential for breeding progress. To further elucidate the mechanisms responsible for cold tolerance, a cold-tolerant and a cold-sensitive parental line, along with their reciprocal F1 hybrids, were subjected to cold stress at various stages of reproductive development, with a focus on pollen fertility and receptivity of female floral organs. For this purpose, pollen measurements were conducted using impedance flow cytometry, and the panicle harvest index was determined post-maturation. While existing literature primarily attributes reduced pollen fertility as the cause of decreased seed set, this study provides evidence that female floral organs might be more affected than previously assumed. We found that the onset of generative tissue formation until BBCH39 (flag leaf visible) is the most cold-sensitive developmental stage and that there is no predominance of maternal or paternal effects associated with the inheritance of cold tolerance in reciprocal F1 hybrids. These findings offer valuable insights for the development of cold-tolerant sorghum varieties to enable cultivation in colder regions and enhance yield stability in temperate climates. Further studies should aim at validating and expanding these findings from the limited number of representative genotypes analyzed in the present manuscript to global sorghum diversity.
Keywords: Sorghum bicolor; climate adaptation; cold sensitivity; pollen fertility; reproductive cold tolerance; sorghum hybrid breeding; spikelet fertility.
© 2025 The Author(s). Plant Direct published by American Society of Plant Biologists and the Society for Experimental Biology and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures








References
-
- Berenji, J. , and Dahlberg J.. 2004. “Perspectives of Sorghum in Europe.” Journal of Agronomy and Crop Science 190, no. 5: 332–338. 10.1111/j.1439-037X.2004.00102.x. - DOI
-
- Bindraban, P. S. , van der Velde M., Ye L., et al. 2012. “Assessing the Impact of Soil Degradation on Food Production.” Current Opinion in Environmental Sustainability 4, no. 5: 478–488. 10.1016/j.cosust.2012.09.015. - DOI
-
- Brooking, I. R. 1976. “Male Sterility in Sorghum bicolor (L.) Moench Induced by Low Night Temperature. I. Timing of the Stage of Sensitivity.” Functional Plant Biology 3, no. 5: 589. 10.1071/PP9760589. - DOI
-
- Caddel, J. L. , and Weibel D. E.. 1971. “Effect of Photoperiod and Temperature on the Development of Sorghum.” Agronomy Journal 63, no. 5: 799–803. 10.2134/agronj1971.00021962006300050043x. - DOI
-
- Casper, B. B. 1990. “Timing of Embryo Abortion and the Effect of Ovule Thinning on Nutlet Mass in Cryptantha flava (Boraginaceae).” Annals of Botany 65, no. 5: 489–492. 10.1093/oxfordjournals.aob.a087960. - DOI
LinkOut - more resources
Full Text Sources

