Comparison of the Safety and Effectiveness of Labor Induction With 25 μg Versus 50 μg of Oral Misoprostol in Women With Premature Rupture of Membranes: A Randomized Controlled Trial
- PMID: 40330768
- PMCID: PMC12051426
- DOI: 10.1002/hsr2.70817
Comparison of the Safety and Effectiveness of Labor Induction With 25 μg Versus 50 μg of Oral Misoprostol in Women With Premature Rupture of Membranes: A Randomized Controlled Trial
Abstract
Background and aims: The recommended dosing regimens for oral misoprostol in labor induction include 25 μg every 2 h and 50 μg every 4 h. However, there is no specific protocol for these regimens at Shahid Akbarabadi Hospital. This study aimed to assess the safety and effectiveness of the two dosing protocols for labor induction in women with premature rupture of membranes (PROM) and establish a tailored protocol to minimize maternal and neonatal complications.
Methods: This randomized controlled trial was conducted at Shahid Akbarabadi Hospital from 2021 to 2023, including pregnant women with term singleton pregnancies and confirmed PROM. Participants were randomly assigned to receive either 25 μg of oral misoprostol every 2 h (up to 12 doses) or 50 μg every 4 h (up to 6 doses). The primary outcome was the mode of delivery. Secondary outcomes included induction-to-delivery time, uterine tachysystole, postpartum hemorrhage, and neonatal complications. Statistical analysis was performed using chi-square and t-tests for comparisons between groups.
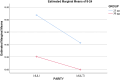
Results: A total of 400 women were enrolled, with 200 in each group. The mean induction-to-delivery interval was significantly shorter in the 50 μg group (p = 0.001). Uterine tachysystole and postpartum hemorrhage due to atonia were more frequent in the 25 μg group (p < 0.05). The rates of cesarean and instrumental deliveries did not differ significantly between groups. The mean ± SD age was 27.1 ± 5.27 years in the 25 μg group and 26.8 ± 5.11 years in the 50 μg group (p = 0.639).
Conclusion: Both dosing regimens of oral misoprostol were effective for labor induction, but the 50 μg dose was associated with a shorter induction-to-delivery time. The findings suggest that adjusting the misoprostol dosage may reduce complications. Clinical Trial Registration Code: IRCT20221123056587N1.
Keywords: childbirth; misoprostol; pregnancy complications.
© 2025 The Author(s). Health Science Reports published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Sharma M., Agrwal M., and Goudappagoudra H., “Maternal and Fetal Outcome in Preterm Premature Rupture of Membrane,” International Journal of Reproduction, Contraception, Obstetrics and Gynecology 10, no. 11 (2021): 4178–4183.
-
- Ismail A. Q. T. and Lahiri S., “Management of Prelabour Rupture of Membranes (PROM) at Term,” Journal of Perinatal Medicine 41, no. 6 (2013): 647–649. - PubMed
-
- Olita'a D., Barnabas R., Vali Boma G., Pameh W., Vince J., and Duke T., “Simplified Management Protocol for Term Neonates After Prolonged Rupture of Membranes in a Setting With High Rates of Neonatal Sepsis and Mortality: A Quality Improvement Study,” Archives of Disease in Childhood 104, no. 2 (2019): 115–120. - PMC - PubMed
-
- Tsakiridis I., Mamopoulos A., Athanasiadis A., and Dagklis T., “Induction of Labor: An Overview of Guidelines,” Obstetrical & Gynecological Survey 75, no. 1 (2020): 61–72. - PubMed
LinkOut - more resources
Full Text Sources