The Median Effective Dose of Ciprofol Combined with Sufentanil for Inhibiting Responses to Gastroscope Insertion in Obese Patients: A Prospective, Single-Center Study
- PMID: 40330816
- PMCID: PMC12051986
- DOI: 10.2147/DDDT.S494972
The Median Effective Dose of Ciprofol Combined with Sufentanil for Inhibiting Responses to Gastroscope Insertion in Obese Patients: A Prospective, Single-Center Study
Abstract
Background: Ciprofol, a recently developed intravenous anesthetic, whereas sufentanil is a widely used adjuvant for gastroenteroscopy sedation. The recommended dosage of ciprofol for obese patients remains unclear. Our study aimed to determine the median effective dose (ED50) of ciprofol in combination with sufentanil for obese patients undergoing gastroscopy sedation.
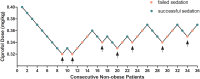
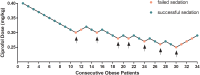
Methods: A total of 70 patients undergoing painless gastroscopy from July 2024 to September 2024 were recruited. Patients were assigned to the obese group (body mass index [BMI]≥28 kg/m2, n=34) and non-obese group (18.5 kg/m2 ≤BMI<24 kg/m2, n=36). All patients received 0.1 μg/kg of sufentanil, and the ciprofol dose was determined by the modified Dixon sequential method with an initial dose of 0.4 mg/kg and a dose gradient of 0.01 mg/kg. The dose of ciprofol administered to the subsequent patient was determined by the response of the preceding patient. The response referred to the patient's cough, swallowing, and body movement during gastroscope insertion. The primary outcome was the ED50 of ciprofol in each group, while the secondary outcomes comprised the incidences of hypoxemia, hypotension, bradycardia, postoperative nausea and vomiting (PONV), and hemodynamic parameters.
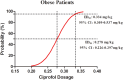
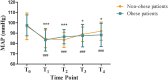
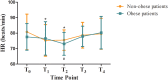
Results: The ED50 of ciprofol was 0.278 mg/kg (95% confidence interval [CI]: 0.226-0.297 mg/kg) in the obese group and 0.347 mg/kg (95% CI: 0.329-0.360 mg/kg) in the non-obese group for gastroscopy sedation. The ED50 of ciprofol in the obese group was significantly lower than that in the non-obese group (P<0.05). The incidence of hypoxemia in the obese group was significantly higher than that in the non-obese group (P<0.05).
Conclusion: Obesity affected the ED50 of ciprofol, suggesting that the ciprofol dosage should be adjusted in obese patients.
Keywords: ciprofol; median effective dose; obesity; painless gastroscopy.
© 2025 Xue et al.
Conflict of interest statement
All authors declare that they have no competing interests.
Figures

Similar articles
-
The median effective dose of ciprofol combined with a low-dose sufentanil for gastroscopy in obese or nonobese patients: a dose-finding study using Dixon's up-and-down method.Front Pharmacol. 2025 Feb 18;16:1521715. doi: 10.3389/fphar.2025.1521715. eCollection 2025. Front Pharmacol. 2025. PMID: 40041487 Free PMC article.
-
Effective dose of ciprofol combined with low-dose sufentanil for sedation of gastroscopy: a dose-finding study using a biased coin design.BMC Anesthesiol. 2025 Mar 14;25(1):124. doi: 10.1186/s12871-025-02986-3. BMC Anesthesiol. 2025. PMID: 40087568 Free PMC article. Clinical Trial.
-
The median effective dose of ciprofol combined with sufentanil in suppressing the laryngeal mask airway insertion response in both young and older adult patients.BMC Anesthesiol. 2024 Dec 19;24(1):464. doi: 10.1186/s12871-024-02855-5. BMC Anesthesiol. 2024. PMID: 39702017 Free PMC article.
-
Effective doses of ciprofol combined with alfentanil in inhibiting responses to gastroscope insertion, a prospective, single-arm, single-center study.BMC Anesthesiol. 2024 Jan 2;24(1):2. doi: 10.1186/s12871-023-02387-4. BMC Anesthesiol. 2024. PMID: 38166724 Free PMC article.
-
A systematic review and meta-analysis comparing the efficacy and safety of ciprofol (HSK3486) versus propofol for anesthetic induction and non-ICU sedation.Front Pharmacol. 2023 Sep 25;14:1225288. doi: 10.3389/fphar.2023.1225288. eCollection 2023. Front Pharmacol. 2023. PMID: 37818194 Free PMC article.
References
-
- Wadhwa V, Issa D, Garg S, Lopez R, Sanaka MR, Vargo JJ. Similar risk of cardiopulmonary adverse events between propofol and traditional anesthesia for gastrointestinal endoscopy: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2017;15(2):194–206. doi:10.1016/j.cgh.2016.07.013 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

