A Comprehensive Review on the Pharmacokinetics and Drug-Drug Interactions of Approved GLP-1 Receptor Agonists and a Dual GLP-1/GIP Receptor Agonist
- PMID: 40330819
- PMCID: PMC12052016
- DOI: 10.2147/DDDT.S506957
A Comprehensive Review on the Pharmacokinetics and Drug-Drug Interactions of Approved GLP-1 Receptor Agonists and a Dual GLP-1/GIP Receptor Agonist
Abstract
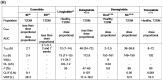
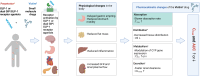
Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) are peptide-derived analogs that were initially investigated to treat type 2 diabetes. Recently, a drug targeting the receptors of both GLP-1 and glucose-dependent insulinotropic polypeptide (GIP) (tirzepatide) has been introduced to the market, and its indications have expanded to include treating obesity. Here, we review the pharmacokinetics, pharmacokinetic drug-drug interactions (DDIs), and pharmacokinetic modeling approaches of four currently available GLP-1 RAs (exenatide, liraglutide, dulaglutide, and semaglutide) and tirzepatide. To address the extremely short half-life (2 min) of native human GLP-1, structural modifications have been applied to GLP-1 RAs and a dual GLP-1/GIP RA. These include amino acid sequence substitutions, fatty acid conjugation using a linker, and fusion with albumin or the IgG fragment crystallizable (Fc) region, resulting in minimal metabolism and renal excretion. Due to their diverse structures, the pharmacokinetic profiles vary, and a prolonged half-life may be associated with an increased risk of adverse events. Clinically significant drug-metabolizing enzyme- and transporter-mediated DDIs are yet to be reported. Mechanism-of-action-mediated DDIs are currently limited to those involving delayed gastric emptying, and most studies have found them to be clinically insignificant. However, significant changes in exposure were observed for oral contraceptives and levothyroxine following the administration of tirzepatide and oral semaglutide, respectively, indicating the need for close monitoring in these instances. Thirty models have been developed to predict pharmacokinetics and physiologically based pharmacokinetic modeling can be useful for assessing mechanism-of-action-mediated DDIs. Alterations in the volume of distribution and clearance resulting from other mechanisms of action (eg, reduced fat mass, changes in cytochrome P450 activity, and glomerular filtration rate) are key factors in determining pharmacokinetics. However, the DDIs mediated by these factors remain poorly understood and require further investigation to ensure that GLP-1 RAs can be safely used with concomitant medications.
Keywords: GIP; GLP-1; drug−drug interactions; pharmacokinetics; physiologically based pharmacokinetic model.
© 2025 Min et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

