Direct Electrosynthesis of an Amino Acid from a Biomass Derivative
- PMID: 40331008
- PMCID: PMC12051197
- DOI: 10.1021/acselectrochem.4c00171
Direct Electrosynthesis of an Amino Acid from a Biomass Derivative
Abstract
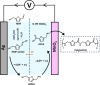
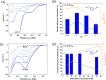
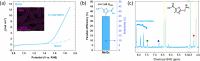
The electrochemical synthesis of nitrogen-containing molecules from biomass-derived compounds under ambient conditions is demonstrated, relying only on green sources of feedstock, renewable energy, and water. In this study, we report a two-step method of electrochemically synthesizing 5-(aminomethyl)furan-2-carboxylic acid (AFCA) from 5-hydroxymethylfurfural (HMF) using hydroxylamine (NH2OH) as the nitrogen source in an acidic electrolyte. In the first step, HMF was reductively aminated into (5-(aminomethyl)furan-2-yl)methanol (HMFA) using NH2OH as the source of nitrogen. This was followed by a second step, involving the oxidation of HMFA to AFCA on a manganese oxide (MnO x ) anode at the same pH. MnO x was able to selectively oxidize the alcohol group on HMFA to produce AFCA with 35% Faradaic efficiency without affecting the amine group. As both of these reactions are completed in a pH 1 electrolyte, it eliminates the need to separate HMFA before proceeding with the second reaction. Among different metal electrodes (Ag, Au, Cu, Pb, Pt and Sn) tested for the electrochemical reductive amination reaction, Ag electrodes displayed the best performance to selectively aminate HMF to the intermediate species, HMFA, with up to 69% Faradaic efficiency at mild potentials of -0.50 VRHE. Density functional theory calculations were carried out to explore a possible reaction pathway for the reductive amination on Ag(111), which suggests a thermodynamically feasible reaction even at 0 VRHE. The cathodic experimental reaction parameters were optimized to reveal that an electrolyte pH of 1 is optimal for the electrochemical reductive amination reaction. Our work shapes the future possibility of an electrochemical synthesis to produce AFCA without the need for any product separation between steps by combining the Ag cathode reaction to the MnO x anode reaction sharing the same electrolyte. Since both the cathode and anode reactions both involve four electrons transferred, combining both half reactions in a single electrochemical reactor can eliminate the need for energy-wasting auxiliary counter reactions such as hydrogen evolution or water oxidation.
© 2025 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Mamman A. S.; Lee J.-M.; Kim Y.-C.; Hwang I. T.; Park N.-J.; Hwang Y. K.; Chang J.-S.; Hwang J.-S. Furfural: Hemicellulose/xylosederived biochemical, Biofuels. Bioprod. Biorefining 2008, 2, 438–454. 10.1002/bbb.95. - DOI
-
- Karimi K.; Kheradmandinia S.; Taherzadeh M. J. Conversion of rice straw to sugars by dilute-acid hydrolysis. Biomass and Bioenergy 2006, 30, 247–253. 10.1016/j.biombioe.2005.11.015. - DOI
-
- Zhang J.; Lin L.; Liu S. Efficient production of furan derivatives from a sugar mixture by catalytic process. Energy Fuels 2012, 26, 4560–4567. 10.1021/ef300606v. - DOI
-
- Kwon Y.; Schouten K. J. P.; Van Der Waal J. C.; De Jong E.; Koper M. T. M. Electrocatalytic Conversion of Furanic Compounds. ACS Catal. 2016, 6, 6704–6717. 10.1021/acscatal.6b01861. - DOI
-
- Hoang A. T.; Ölçer A. I.; Nižetić S. Prospective review on the application of biofuel 2,5-dimethylfuran to diesel engine. J. Energy Inst. 2021, 94, 360–386. 10.1016/j.joei.2020.10.004. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
