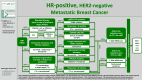
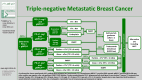
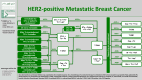
AGO Recommendations for the Diagnosis and Treatment of Patients with Locally Advanced and Metastatic Breast Cancer: Update 2025
- PMID: 40331128
- PMCID: PMC12052354
- DOI: 10.1159/000545018
AGO Recommendations for the Diagnosis and Treatment of Patients with Locally Advanced and Metastatic Breast Cancer: Update 2025
Abstract
The Breast Committee of the Arbeitsgemeinschaft Gynäkologische Onkologie (AGO; German Gynecological Oncology Group) presents the 2025 update of the evidence-based recommendations for the diagnosis and treatment of patients with locally advanced and metastatic breast cancer (mBC).
Keywords: AGO; Diagnosis; Metastatic breast cancer; Recommendations; Treatment.
© 2025 S. Karger AG, Basel.
Conflict of interest statement
Prof. Dr. med. Marc Thill is in the advisory boards of Agendia, Amgen, AstraZeneca, AURIKAMED, Becton/Dickinson, Biom’Up, ClearCut, Clovis, Daiichi Sankyo, Eisai, Exact Sciences, Gilead Science, Grünenthal, GSK, Johnson & Johnson, Lilly, MSD, Neodynamics, Novartis, onkowissen, Organon, Pfizer, pfm Medical, Pierre Fabre, Roche, RTI Surgical, SamanTree, Seagen, Sirius Medical, and Sysmex and has received manuscript support from Amgen, CairnSurgical ClearCut, Clovis, Lilly, Organon, pfm medical, Roche, and Servier; travel expenses from Amgen, Art Tempi, AstraZeneca, Clearcut, Clovis, Connectmedica, Daiichi Sankyo, Eisai, Exact Sciences, Gilead, Hexal, I-MED Institute, Lilly, Menarini Stemline, MSD, Neodynamics, Novartis, Pfizer, pfm Medical, Roche, RTI Surgical, Seagen, and ZP Therapeutics; congress support from Amgen, AstraZeneca, Celgene, Daiichi Sanyko, Gilead, Hexal, Lilly, Menarini Stemline, Neodynamics, Novartis, Pfizer, pfm medical, Pierre Fabre, Roche, and Sirius Medical; lecture honoraria from Agendia, Amgen, Art Tempi, AstraZeneca, Clovis, Connectmedica, Eisai, Endomag, Exact Sciences, Gedeon Richter, Gilead Science, GSK, Hexal, I-MED Institute, Jörg Eickeler, Laborarztpraxis Walther et al. Lilly, Medscape, Menarini Stemline, MSD, Novartis, onkowissen, Pfizer, pfm medical, Roche, Seagen, Sirius Medical, STREAMED UP, Sysmex, Vifor, Viatris, and ZP Therapeutics; trial funding from Endomag and Exact Sciences; and trial honoraria (institutional) from AstraZeneca, Biom’Up, CairnSurgical, Clearcut, Neodynamics, Novartis, pfm medical, Roche, and RTI Surgical. Prof. Dr. med. Wolfgang Janni has received research grants and/or honoraria from AstraZeneca, Celgene, Chugai, Daiichi Sankyo, Eisai, Exact Sciences, GSK, Janssen, Lilly, Menarini, MSD, Novartis, Sanofi-Aventis, Roche, Pfizer, Seagen, Gilead, Inivata, and Guardant Health. Prof. Dr. med. Ute-Susann Albert has received lectures from Pfizer, Novartis, and AstraZeneca and is in the advisory boards of Daiichi Sankyo and Pfizer. Prof. Dr. Maggie Banys-Paluchowski has received honoraria for lectures and advisory from Roche, Novartis, Pfizer, pfm, Eli Lilly, onkowissen, Seagen, AstraZeneca, Eisai, Amgen, Samsung, Canon, MSD, GSK, Daiichi Sankyo, Gilead, Sirius Medical, Syantra, resitu, Pierre Fabre, and Exact Sciences; trial support from Korean Breast Cancer Society, EndoMag, Mammotome, Merit Medical, Sirius Medical, Gilead, Hologic, Exact Sciences, Claudia von Schilling Stiftung, Damp Stiftung, and Ehmann Stiftung Savognin; travel expenses and congress support from Eli Lilly, Exact Sciences, Pierre Fabre, Pfizer, Daiichi Sankyo, Roche, and Stemline. Prof. Dr. med. Rupert Bartsch has performed an advisory role at AstraZeneca, Daiichi, Eisai, Eli Lilly, Gilead, Grunenthal, MSD, Novartis, Pfizer, Pierre Fabre, Roche, Seagen, and Stemline; received lecture honoraria from Amgen, AstraZeneca, BMS, Daichi, Eisai, Eli Lilly, Gilead, Grunenthal, MSD, MedMedia, Novartis, Pfizer, Pierre Fabre, Roche, Seagen, and Stemline; received travel support from AstraZeneca, Daiichi, MSD, and Novartis; research support from Daiichi. Dr. med. Ingo Bauerfeind has been a member of the Breast Cancer Germany Association, Kempten Hospital, Academy for Psycho-Oncology, IF Congress Management. Prof. Dr. med. Vesna Bjelic-Radisic has received Adboard honoraria from Pfizer, Roche, Lilly, Stemline, and pfm; lecture honoraria from Pfizer, Lilly, Stemline, pfm medical, and mammotome; travel support from Pfizer. Prof. Dr. med. Jens-Uwe Blohmer has received honoraria for advisory boards and lectures from AstraZeneca, Daiichi Sankyo, Eisai, Gilead, Lilly, MSD, Novartis, Pfizer, Roche, and Seagen. Prof. Dr. med. Wilfried Budach has received honoraria for lectures and advisory boards from Merck, BMS, Jörg Eickeler Veranstaltungen, medpublico GmbH, and BVDST. Prof. Dr. med. Peter Dall has received honoraria for lectures and advisory boards from Novartis, Pierre Fabré, MSD, Lilly, AstraZeneca, Gilead, Daiichi Sankyo, Eisai, and Polytech. Prof. Dr. Nina Ditsch has been a member of advisory boards and speakers bureaus of AstraZeneca, AURIKAMED, BGGF, Daiichi Sankyo, Elsevier Verlag, ESO, Exact Sciences, Gilead Sciences, GSK, if-kongress, KelCon, Leopoldina Schweinfurt, Lilly, Lukon, Molecular Health, MSD, Novartis, onkowissen, Pfizer, RG-Ärztefortbildungen, Roche, and Seagen. Prof. Dr. med. Eva Maria Fallenberg has received a research grant from DFG; speaker honoraria from GE Healthcare, Bayer Healthcare, Guerbet, Siemens, BD, Roche, EUSOBI, ESOR, ESMO, and B-Rayz. Prof. Dr. med. Peter A. Fasching participated on a data safety monitoring board or advisory boards of Novartis, Pfizer, Roche, Daiichi Sankyo, AstraZeneca, Lilly, Eisai, Merck Sharp & Dohme, Pierre Fabre, SeaGen, Agendia, Sanofi-Aventis, Gilead, and Mylan and has received honoraria for lecture, speakers bureaus, manuscript writing, consulting, or educational events from Novartis, Pfizer, Roche, Daiichi Sankyo, AstraZeneca, Lilly, Eisai, Merck Sharp & Dohme, Pierre Fabre, SeaGen, Agendia, Sanofi-Aventis, Gilead, and Mylan; medical writing for Merck; and has contributed to institution at BioNTech, Cepheid, and Pfizer. Prof. Dr. med. Tanja N. Fehm has received support from onkowissen. Prof. Dr. med. Michael Friedrich has been in advisory board of Gilead Sciences and has received other honoraria from Roche and MSD. Prof. Dr. med. Bernd Gerber has received travel support from Pfizer. PD Dr. med. Oleg Gluz has received honoraria for lectures and/or consulting from Amgen, AstraZeneca, Daiichi Sanyko, Eisai, Gilead Science, Lilly, MSD, Novartis, Pfizer, Roche, Seagen, Exact Sciences, Agendia, MedConcept, and GynUpdate; is a minority shareholder of Westdeutsche Studiengruppe (WSG). Prof. Nadia Harbeck, MD has received honoraria for lectures and/or consulting from AstraZeneca, Daiichi Sankyo, Gilead, Lilly, MSD, Novartis, Pierre Fabre, Pfizer, Roche, Seagen, Viatris, Zuellig, and Pharma, and has been Co-Director West German Study Group (WSG). Prof. Dr. med. Andreas Daniel Hartkopf has received honoraria for consulting and speaking engagements from AstraZeneca, Agendia, Amgen, Clovis, DaichiiSankyo, Eisai, Exact Sciences, Gilead, GSK, Hexal, Lilly, MSD, Novartis, onkowissen, Pfizer, Roche, Pierre Fabre, Seagen, Stemline, and Veracyte. Prof. Dr. med. Jörg Heil has nothing to disclose. Prof. Dr. med. Jens Huober has received honoraria for lectures at Lilly, Novartis, Roche, Pfizer, AstraZeneca, MSD, Seagen, Gilead, and Daiichi; and honoraria for consulting/advisory board at Lilly, Novartis, Roche, Pfizer, AstraZeneca, MSD, Daiichi, Gilead. Travel grants: Roche, Pfizer, Daiichi, and Gilead. Prof. Dr. med. Hans-Heinrich Kreipe has been a member of advisory board of Lilly; and have received lecture honoraria from AstraZeneca, Roche, Daiichi Sankyo, and Pfizer. PD Dr. David Krug has received lecture honoraria from Merck Sharp & Dohme, Pfizer, AstraZeneca, onkowissen, and medupdate; has been a member of advisory board at Gilead; has received research funding from Merch KGaA and Deutsche Krebshilfe. Prof. Dr. med. Thorsten Kühn has been a member of advisory board/lecture at Sysmex, Neodynamics, Pfizer, MSD, Merit Medical, Sirius Medical, Hologic, Endomag, and Lilly and has received trial funding from Merit Medical, Endomag, and Mammotome. Prof. Dr. med. Sherko Kümmel has received lecture honoraria from Roche, Lilly, Exact Sciences, Novartis, Amgen, Daiichi Sankyo, AstraZeneca, MSD, Pfizer, Seagen, Gilead, Agendia; Hologic, PINK!, and Stemline; other honoraria from Roche, Daiichi Sankyo, and Sonoscape; and has been on advisory boards of Lilly, MSD, Roche, AstraZeneca, Stemline, Novartis, Daiichi Sankyo, MSD, Seagen, Gilead, and Exact Sciences. Prof. Dr. med. Sibylle Loibl was a member of advisory board, institutional, of AbbVie, Amgen, AstraZeneca, BMS, Celgene, DSI, Eirgenix, GSK, Gilead Science, Lilly, Novartis, Olema, Pfizer, Pierre Fabre, Relay Therapeutics, Puma, Roche, Seagen, and Stemline-Menarini; was an invited speaker, personal, at Medscape; has received trial funding/others from AstraZeneca, AbbVie, Celgene, Daiichi Sankyo, Greenwich Life Sciences, GSK, Immunomedics/Gilead, Molecular Health, Novartis, Pfizer, Roche, Stemline-Menarini, and VM Scope GmbH; has received grants and/or honorarium for adboards and/or contracts from the following entities/all paid to institution: AZ, AbbVie, Agendia, Amgen, BionTech, Celgene/BMS, Celcuity, DSI, Exact Sciences, Gilead, GSK, Incyte, Lilly, Medscape, Molecular Health, MSD, Novartis, Pierre Fabre, Pfizer, Relay, Roche, Sanofi, Seagen, Stemline/Menarini, Olema, Bayer, Bicycle, JAZZ Pharma, and BeiGene; received support for attending meetings and/or travel from DSI, ESMO, SGBCC, ASCO, AGO Kommission Mamma, Patents planned, issued, or pending: EP14153692.0/EP21152186.9/EP18209672/EP24210258; and has received royalties from VM Scope. Prof. Dr. med. Diana Lüftner has received honoraria for advisory board activities and/or oral presentations from Amgen, AstraZeneca, Daiichi Sankyo, Eli Lilly, Gilead, GSK, high5md, Loreal, MSD, Mundipharma, Novartis, onkowissen.de, Pfizer, Pierre Fabre, Roche, and TEVA. Prof. Dr. med. Michael Patrick Lux has received honoraria from Lilly, Pfizer, Roche, MSD, Hexal, Novartis, AstraZeneca, Eisai, Exact Sciences, Agendia, Daiichi Sankyo, Grünenthal, Gilead, Pierre Fabre, PharmaMar, SamanTree, Endomag, and medac for advisory boards, lectures, and travel support. Prof. Dr. med. Nicolai Maass has been on the advisory board of Amgen, AstraZeneca, Clovis, Daiichi Sankyo, GSK, Lilly, MSD, Novartis, Pierre Fabre, Pfizer, Roche, and Seagen and has received lecture honoraria from AstraZeneca, Daiichi Sankyo, GSK, Lilly, Novartis, Pfizer, and Roche. Prof. Dr. med. Christoph Mundhenke has been a member of advisory boards of AstraZeneca, Pfizer, Daiichi Sankyo, Seagen, and Novartis and received lecture honoraria from Pfizer and Novartis. Prof. Dr. med. Toralf Reimer has received trial funding from German Cancer Aid and Else Kroener-Fresenius-Stiftung; has been on advisory boards of MSD, Novartis, and Myriad; and has received lecture honoraria from Pfizer, Novartis, Roche, and AstraZeneca. Prof. Dr. med. Kerstin Rhiem has received support from AstraZeneca, Roche, Novartis, and Streamed Up. PD Dr. med. Mattea Reinisch has received honoraria and/or consultancy fee from AstraZeneca, Daiichi Sankyo, Gilead, Lilly, MSD, Novartis, Pfizer, Roche, Seagen, Streamed Up, and SOMATEX and travel support from AstraZeneca, Daiichi Sankyo, Gilead, Lilly, Novartis, Pfizer, and Roche. Prof. Dr. med. Achim Rody has been on advisory boards of AstraZeneca, Novartis, Roche, Exact Sciences, Pierre Fabre, Lilly, Seagen, Amgen, MSD, and Gilead; has received lecture honoraria from Pfizer, Celgene, and Eisai; and has received trial funding from Eisai. Prof. Dr. med. Marcus Schmidt reports personal fees from AstraZeneca, BioNTech, Daiichi Sankyo, Eisai, GILEAD, Lilly, Menarini Stemline, Molecular Health, MSD, Novartis, Pantarhei Bioscience, Pfizer, Pierre Fabre, Roche, and SeaGen; his institution has received research funding from AstraZeneca, BioNTech, Eisai, Genentech, German Breast Group, Novartis, Palleos, Pantarhei Bioscience, Pierre Fabre, and SeaGen. In addition, he has a patent for EP 2390370 B1 and a patent for EP 2951317 B1 issued. Prof. Dr. med. Andreas Schneeweiss has received research grants from Celgene and Roche; honoraria from Amgen, AstraZeneca, AURIKAMED, Bayer, Celgene, ClinSol, Clovis Oncology, coma UroGyn, Connectmedica, Daiichi Sankyo, Gilead, GSK, if-kongress, I-MED, iOMEDICO, Lilly, MCI Deutschland, medpublico, Metaplan, MSD, Mylan, NanoString Technologies, Novartis, onkowissen.de, Pfizer, Pierre Fabre, promedicis, Roche, Seagen, STREAMED UP, and Tesaro; and travel support from AstraZeneca, Celgene, Daiichi Sankyo, Gilead, Pfizer, and Roche. Prof. Dr. med. Florian Schütz has received lecture honoraria from Amgen, AstraZeneca, Daiichi Sankyo, Eisai, Exact Sciences, Gilead, Lilly, MSD, Novartis, ClinSol, Pfizer, and Roche Pharma; has been on advisory boards of Lilly, MSD, Gilead, Atheneum Partners, and ClinSol; has received travel expenses from Lilly and Gilead. Prof. Dr. med. Hans-Peter Sinn has been on advisory boards of AstraZeneca, Exact Sciences, and Daiichi Sankyo; has received lecture honoraria from AstraZeneca and Acentus; and has received trial funding from AstraZeneca. Prof. Dr. med. Christine Solbach has received lecture honoraria from DiaLog Service GmbH, Jörg Eickeler, Pfizer, Roche, AstraZeneca, MedConcept, I-MED, GBG, BVF Akademie, and LÄK Hessen Akademie and has been on advisory boards of MSD and Roche. Prof. Dr. med. Erich-Franz Solomayer has received support from Roche, Amgen, Celgen, Tesaro, AstraZeneca, Pfizer, Storz, Erbe, Gedeon Richter, Eisai, Medac, MSD, Vifor, Teva, Ethicon, Johnson & Johnson, Daiichi Sankyo, Gilead, Exact Sciences, GSK, and Pierre Fabre. Prof. Dr. med. Elmar Stickeler has been on advisory boards of Amgen, AstraZeneca, Gilead, iOMEDICO, Lilly, MSD, Novartis, Seagen, and Roche; has received lecture honoraria from AstraZeneca, BSH Düsseldorf, Gilead, iOMEDICO, MSD, Novartis, onkowissen, Pfizer, PharmaMar, and Roche. Prof. Dr. med. Christoph Thomssen received compensation for advisory boards, lectures, or publications from Amgen, AstraZeneca, AURIKAMED, Daiichi Sankyo, Forum Sanitas, Gilead, Jörg Eickeler, Hexal, Lilly, medupdate, MSD, Nanostring, Novartis, onkowissen, Pfizer, Roche, Seagen, and Vifor. Prof. Dr. med. Michael Untch has received honoraria to travel support, lectures, and consulting or advisory role from AstraZeneca, Amgen, Daiichi Sankyo, Lilly, Roche, Pfizer, MSD Oncology, Pierre Fabre, Sanofi-Aventis, Myriad, Seagen, Novartis, Gilead, Stemline, Genzyme, Agendia, onkowissen, and Eisai, all honoraria and fees to the employer/institution. Prof. Dr. med. Marion van Mackelenbergh has an advisory role at Daiichi Sankyo, Exact Sciences, Gilead, Novartis, Pfizer, Pierre Fabre, and Roche; has received lecture honoraria from AstraZeneca, Daiichi Sankyo, Elsevier, GSK, Jenapharm, Lilly, Menarini, Novartis, Pfizer, and Seagen; and travel support from Daiichi Sankyo and Gilead. Prof. Dr. Isabell Witzel has received lecture honoraria from AstraZeneca, Lilly, Seagen, Daiichi Sankyo, Gilead, Pfizer, Novartis, and onkowissen; and has received travel support from Roche and Lilly. Prof. Dr. med. Achim Wöckel has received compensation for advisory boards, lectures, trial funding, advisory role from Amgen, AstraZeneca, AURIKAMED, Celgene, Eisai, Lilly, Novartis, Pfizer, Roche, Tesaro, Sirtex, MSD, Exact Sciences, Pierre Fabre, Clovis, Organon, Daiji Sankyo, Seagen, Stemline, and Gilead. PD. Dr. Rachel Würstlein served as advisor, consultant, speaker, and travel grant at Agendia, Amgen, Apogepha, Aristo, AstraZeneca, Celgene, Clovis Oncology, Daiichi Sankyo, Eisai, Esteve, Exact Sciences, Gilead, Glaxo Smith Kline, Hexal, Lilly, Medstrom Medical, MSD, Mundipharma, Mylan, Nanostring, Novartis, Odonate, Paxman, Palleos, Pfizer, Pierre Fabre, PINK, PumaBiotechnolgogy, RIEMSER, Roche, Sandoz/Hexal, Sanofi Genzyme, Seattle Genetics/Seagen, Sidekick, Stemline, Tesaro Bio, Teva, Veracyte, Viatris, Wiley, FOMF, AURIKAMED, Clinsol, Pomme Med, medconcept, MCI, and MediSeminar. Prof. Dr. med. Volkmar Müller has received speaker honoraria from AstraZeneca, Daiichi Sankyo, Eisai, Pfizer, MSD, Medac, Novartis, Roche, Seagen, onkowissen, high5 Oncology, Medscape, Gilead, Pierre Fabre, and I-MED Institute; consultancy honoraria from Roche, Pierre Fabre, PINK, ClinSol, Novartis, MSD, Daiichi Sankyo, Eisai, Lilly, Seagen, Gilead, and Stemline; institutional research support from Novartis, Roche, Seagen, Genentech, and AstraZeneca; and travel grants from AstraZeneca, Roche, Pfizer, Daiichi Sankyo, and Gilead. Prof. Dr. Tjoung-Won Park-Simon has received honoraria for lectures and/or consulting from Roche, AstraZeneca, GSK, Pfizer, Lilly, MSD, Exact Sciences, Daiichi Sankyo, Seagen, Novartis, Gilead Science, NCO, onkowissen, Exact Sciences, and Seagen and travel compensation from Roche, AstraZeneca, Pfizer, Lilly, Daiichi Sankyo, Gilead, and Pierre Fabre.
Figures
References
-
- Empfehlungen Gynäkologische Onkologie kommission Mamma. 2025; www.ago-online
-
- Papakonstantinou A, Gonzalez NS, Pimentel I, Suñol A, Zamora E, Ortiz C, et al. Prognostic value of ctDNA detection in patients with early breast cancer undergoing neoadjuvant therapy: a systematic review and meta-analysis. Cancer Treat Rev. 2022;104:102362. - PubMed
-
- Bidard FC, Hardy-Bessard AC, Dalenc F, Bachelot T, Pierga JY, de la Motte Rouge T, et al. Switch to fulvestrant and palbociclib versus no switch in advanced breast cancer with rising ESR1 mutation during aromatase inhibitor and palbociclib therapy (PADA-1): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2022;23(11):1367–77. - PubMed
-
- Bidard FC, Kaklamani VG, Neven P, Streich G, Montero AJ, Forget F, et al. Elacestrant (oral selective estrogen receptor degrader) versus standard endocrine therapy for estrogen receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: results from the randomized phase III EMERALD trial. J Clin Oncol. 2022;40(28):3246–56. - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources