GluN2B influences the progression of status epilepticus by modulating calcium ion homeostasis through its interaction with CaMKIIα
- PMID: 40331194
- PMCID: PMC12053154
- DOI: 10.3389/fphar.2025.1550879
GluN2B influences the progression of status epilepticus by modulating calcium ion homeostasis through its interaction with CaMKIIα
Abstract
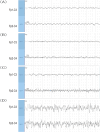
Background: Status epilepticus (SE) is a neurological emergency characterized by prolonged, unresolved epileptic seizures, often resulting in adverse outcomes. Conventional pharmaceuticals are not universally effective in terminating epileptic seizures; therefore, identifying novel targets for seizure cessation and the prevention of SE is crucial. This study aimed to assess the expression levels and interactions of the N-methyl-D-aspartate receptor (NMDAR) subunit GluN2B and CaMKIIα following epileptic convulsions and to explore their potential mechanisms of action.
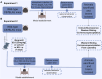
Methods: This study utilized Western blotting to evaluate the protein expression levels of CaMKIIα, p-CaMKIIα, and GluN2B in the hippocampus of mice subjected to kainic acid-induced SE. Immunofluorescence colocalization analysis and co-immunoprecipitation were utilized to investigate the interaction between GluN2B and CaMKIIα in the hippocampus. Additionally, flow cytometry was employed to measure intracellular calcium ion levels.
Results: Compared to the sham operation group, the intracellular calcium ion concentration in the hippocampus of SE mice was elevated, whereas the expression of p-CaMKIIα was markedly reduced. The levels of CaMKIIα and GluN2B remained unchanged, and the immune complex of GluN2B and CaMKIIα in the SE group exhibited a significant increase. The GluN2B inhibitor ifenprodil was found to prolong the latency of epileptic seizures, counteract calcium influx, and modulate the expression of p-CaMKIIα, as well as the immune complex levels of GluN2B and CaMKIIα. These findings suggest that the interaction between GluN2B and CaMKIIα may be critical in the pathophysiological processes of SE, influencing the levels of p-CaMKIIα and calcium ion homeostasis.
Conclusion: The reduction in CaMKIIα phosphorylation levels depends on the NMDAR pathway. When GluN2B binds to CaMKIIα, it may occupy the autophosphorylation site of CaMKIIα (T286 binding site), thereby affecting its autophosphorylation. This results in decreased phosphorylation levels, disruption of NMDAR-dependent calcium homeostasis, and alteration of the excitation/inhibition balance.
Keywords: Camkiiα; GluN2B; calcium homeostasis; ifenprodil; phosphorylation; status epilepticus.
Copyright © 2025 Zhang, Meng, Liu, Wei, Lu, Zheng, Zou and Wu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Ashpole N. M., Song W., Brustovetsky T., Engleman E. A., Brustovetsky N., Cummins T. R., et al. (2012). Calcium/calmodulin-dependent protein kinase Ii (Camkii) inhibition induces neurotoxicity via dysregulation of glutamate/calcium signaling and Hyperexcitability. J. Biol. Chem. 287 (11), 8495–8506. 10.1074/jbc.M111.323915 - DOI - PMC - PubMed
-
- Avalos-Fuentes A., Albarrán-Bravo S., Loya-Lopéz S., Cortés H., Recillas-Morales S., Magaña J. J., et al. (2015). Dopaminergic Denervation Switches Dopamine D3 receptor signaling and disrupts its Ca(2+) dependent modulation by Camkii and calmodulin in Striatonigral Projections of the rat. Neurobiol. Dis., 74336–74346. 10.1016/j.nbd.2014.12.008 - DOI - PubMed
LinkOut - more resources
Full Text Sources

