Removing Barriers to Tumor 'Oxygenation': Depleting Glutathione Nanozymes in Cancer Therapy
- PMID: 40331231
- PMCID: PMC12051984
- DOI: 10.2147/IJN.S515734
Removing Barriers to Tumor 'Oxygenation': Depleting Glutathione Nanozymes in Cancer Therapy
Abstract
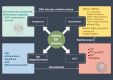
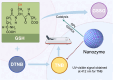
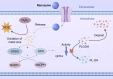
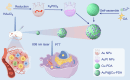
Nanozymes are nanomaterials capable of mimicking natural enzyme catalysis in the complex biological environment of the human body. Due to their good stability and strong catalytic properties, nanozymes are widely used in various fields of biomedicine. Among them, nanozymes that trigger intracellular reactive oxygen species (ROS) levels for cancer therapy have gained significant attention. However, the 'explosion' of ROS in tumor cells was prevented by the high levels of glutathione (GSH) in the tumor microenvironment (TME). GSH, a prominent endogenous antioxidant, increases the resistance of tumor cells to oxidative stress by scavenging ROS. Certain nanozymes can deplete intracellular GSH levels by mimicking GSH oxidase (GSHOx), GSH peroxidase (GPx) or by interfering with the reduction of oxidized glutathione (GSSG). On the one hand, elevated the level of intracellular ROS and induced lipid peroxidation reaction leading to ferroptosis. On the other hand, it creates favorable conditions for the treatment of tumors with photodynamic therapy (PDT), sonodynamic therapy (SDT), chemodynamical therapy (CDT) and targeted therapy. In this paper, we present a comprehensive analysis of GSH-depleting nanozymes reported in recent years, including classification, mechanism, responsiveness to TME and their roles in cancer therapy, and look forward to future applications and developments.
Keywords: cancer therapy; enzyme mimetic; glutathione depletion; nanomaterials; nanozymes; oxygen-dependent therapy.
© 2025 Sun et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

