Adaptations and Heterogeneity of Treatment Effects in Platform Trials-Protocol for Two Methodological Studies
- PMID: 40331329
- PMCID: PMC12056685
- DOI: 10.1111/aas.70044
Adaptations and Heterogeneity of Treatment Effects in Platform Trials-Protocol for Two Methodological Studies
Abstract
Background: Adaptive platform trials bring opportunities for improved infrastructure and effective advancement in medical care but are methodologically complex. Assessment of heterogeneity of treatment effects (HTE) according to participant characteristics and adaptations, including adaptive stopping, are important methodological features in these trials, which may be approached in multiple ways. We aim to characterise the assessment of HTE and use of adaptations, including their key methodological features, in adaptive platform trials.
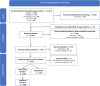
Methods: This protocol outlines two methodological studies, which will be based on a common, systematic literature search and data extraction. We will include adaptive platform trials conducted from 2005 onwards. Screening and data extraction will be performed independently and in duplicate. In Study I, we will assess methods used to evaluate HTE, and in Study II, we will assess adaptations and stopping rules in the included trials.
Discussion: The two proposed methodological studies will provide an overview of important methodological features regarding the assessment of HTE and adaptations used in adaptive platform trials. Better knowledge of available methods to assess these features can improve the conditions for designing adaptive platform trials and identify areas for further development.
Keywords: adaptive analyses; adaptive platform trial; heterogeneity of treatment effects.
© 2025 The Author(s). Acta Anaesthesiologica Scandinavica published by John Wiley & Sons Ltd on behalf of Acta Anaesthesiologica Scandinavica Foundation.
Conflict of interest statement
T.S.M., A.K.G.J., A.P., M.H.M., and A.G. are involved in the design and conduct of the adaptive
Figures
References
-
- Granholm A., Jensen A. K. G., Lange T., Perner A., Møller M. H., and Kaas‐Hansen B. S., “Designing and Evaluating Advanced Adaptive Randomised Clinical Trials: A Practical Guide,” arXiv (2025): 2501.08765, 10.48550/arXiv.2501.08765. - DOI
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

