Constant pH Molecular Dynamics Simulation of pH Effects on Amyloid-β Structure, Dynamics, and Metal-Binding
- PMID: 40331332
- PMCID: PMC12172581
- DOI: 10.1002/chem.202500547
Constant pH Molecular Dynamics Simulation of pH Effects on Amyloid-β Structure, Dynamics, and Metal-Binding
Abstract
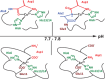
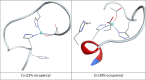
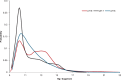
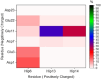
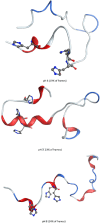
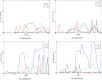
We report the first molecular dynamics simulations to examine the effect of pH on the structure, dynamics, and metal-binding ability of amyloid-β, the peptide implicated in the onset of Alzheimer's disease. We show that in the pH range of 6 to 8 only histidine residues show variable protonation, that predicted pKa values are in agreement with experimental data, and that changes in pH affect the size, flexibility, and secondary structure of the peptide. The binding of Cu(II) or Zn(II) to the peptide induces a shift of 1 to 1.5 pKa units in unbound histidine residues, while metal binding modes associated with higher pH induce significant changes in peptide structure. We speculate on the significance of these findings on results showing pH dependence as well as on Cu(II) and Zn(II) modulation of aggregation of Amyloid-β.
Keywords: amyloid‐β; constant pH; copper; molecular dynamics; secondary structure; zinc.
© 2025 The Author(s). Chemistry – A European Journal published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- a) Weibull M. G. M., Simonsen S., Oksbjerg C. R., Tiwari M. K., Hemmingsen L., J. Biol. Inorg. Chem. 2019, 24, 1197; - PubMed
- b) Atrian‐Blasco E., Conte‐Daban A., Hureau C., Dalton Trans. 2017, 46, 12750; - PMC - PubMed
- c) Yi Y., Lim M. H., RSC Chem. Biol. 2023, 4, 121; - PMC - PubMed
- d) Abelein A., Acc. Chem. Res. 2023, 56, 2653. - PMC - PubMed
-
- Baptista A. M., Martel P. J., Petersen S. B., Proteins. 1997, 27, 523. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

