Clinical staging to guide management of metabolic disorders and their sequelae: a European Atherosclerosis Society consensus statement
- PMID: 40331343
- PMCID: PMC12500331
- DOI: 10.1093/eurheartj/ehaf314
Clinical staging to guide management of metabolic disorders and their sequelae: a European Atherosclerosis Society consensus statement
Erratum in
-
Correction to: Clinical staging to guide management of metabolic disorders and their sequelae: a European Atherosclerosis Society consensus statement.Eur Heart J. 2025 Jun 13:ehaf431. doi: 10.1093/eurheartj/ehaf431. Online ahead of print. Eur Heart J. 2025. PMID: 40512581 No abstract available.
Abstract
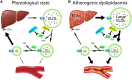
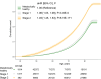
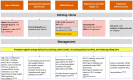
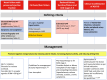
Obesity rates have surged since 1990 worldwide. This rise is paralleled by increases in pathological processes affecting organs such as the heart, liver, and kidneys, here termed systemic metabolic disorders (SMDs). For clinical management of SMD, the European Atherosclerosis Society proposes a pathophysiology-based system comprising three stages: Stage 1, where metabolic abnormalities such as dysfunctional adiposity and dyslipidaemia occur without detectable organ damage; Stage 2, which involves early organ damage manifested as Type 2 diabetes, asymptomatic diastolic dysfunction, metabolic-associated steatohepatitis (MASH), and chronic kidney disease (CKD); and Stage 3, characterized by more advanced organ damage affecting multiple organs. Various forms of high-risk obesity, driven by maintained positive energy balance, are the most common cause of SMD, leading to ectopic lipid accumulation and insulin resistance. This progression affects various organs, promoting comorbidities such as hypertension and atherogenic dyslipidaemia. Genetic factors influence SMD susceptibility, and ethnic disparities in SMD are attributable to genetic and socioeconomic factors. Key SMD features include insulin resistance, inflammation, pre-diabetes, Type 2 diabetes, MASH, hypertension, CKD, atherogenic dyslipidaemia, and heart failure. Management strategies involve lifestyle changes, pharmacotherapy, and metabolic surgery in severe cases, with emerging treatments focusing on genetic approaches. The staging system provides a structured approach to understanding and addressing the multi-faceted nature of SMD, which is crucial for improving health outcomes. Categorization of SMD abnormalities by presence and progression is aimed to improve awareness of a multi-system trait and encourage a tailored and global approach to treatment, ultimately aiming to reduce the burden of obesity-related comorbidities.
Keywords: Heart failure; Insulin resistance/pre-diabetes; Kidney disease; MASLD; Obesity; Type 2 diabetes.
© The Author(s) 2025. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures



References
-
- Phelps NH, Singleton RK, Zhou B, Heap RA, Mishra A, Bennett JE, et al. Worldwide trends in underweight and obesity from 1990 to 2022: a pooled analysis of 3663 population-representative studies with 222 million children, adolescents, and adults. Lancet 2024;403:1027–50. 10.1016/S0140-6736(23)02750-2 - DOI - PMC - PubMed
-
- Ndumele CE, Neeland IJ, Tuttle KR, Chow SL, Mathew RO, Khan SS, et al. A synopsis of the evidence for the science and clinical management of cardiovascular-kidney-metabolic (CKM) syndrome: a scientific statement from the American Heart Association. Circulation 2023;148:1636–64. 10.1161/CIR.0000000000001186 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

