Intraoperative Clonidine in Spine Surgery: A Randomised Controlled Trial
- PMID: 40331364
- PMCID: PMC12056682
- DOI: 10.1111/aas.70048
Intraoperative Clonidine in Spine Surgery: A Randomised Controlled Trial
Abstract
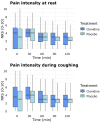
Patients undergoing spine surgery often experience post-operative pain. In this context, clonidine, an alpha-2 agonist, may be relevant due to its analgesic properties. We conducted a randomised, double-blinded, placebo-controlled trial to evaluate the effect of a single dose of intraoperative intravenous clonidine on post-operative opioid consumption, pain intensity and side effects. Patients undergoing spine surgery at Aarhus University Hospital, Denmark, were randomised to receive intraoperative clonidine (3 μg/kg) or placebo. The primary outcome was opioid consumption within the first 3 h after surgery. Secondary outcomes included opioid consumption within the first 6 h, pain intensity at rest and during coughing, post-operative nausea and vomiting (PONV), and sedation in the post-anaesthesia care unit (PACU). Additional outcomes included time to discharge from the PACU, length of hospital stay and daily opioid consumption after 1 month. Data from 120 patients (49 females, 71 males, mean age 65 ± 14 years) were available for analysis; 61 received clonidine and 59 received placebo. Post-operative intravenous morphine equivalents within 3 h were similar in the clonidine group 5 mg (0-15) and the placebo group 10 mg (0-15) (p = 0.58). Pain intensity at rest was 4 (0-5.5) in the clonidine group and 3 (0-5) in the placebo group upon arrival at the PACU (p = 0.20). No differences were observed between the clonidine and placebo groups regarding any secondary outcomes, except for hypotension, which was more frequent in the clonidine group (24 vs. 13 patients). A single dose of intraoperative clonidine did not reduce post-operative opioid consumption or pain intensity in patients undergoing spine surgery.
Keywords: intraoperative clonidine; postoperative pain; spine surgery.
© 2025 The Author(s). Acta Anaesthesiologica Scandinavica published by John Wiley & Sons Ltd on behalf of Acta Anaesthesiologica Scandinavica Foundation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Rasmussen A. M., Toft M. H., Awada H. N., et al., “Waking Up in Pain: A Prospective Unselected Cohort Study of Pain in 3702 Patients Immediately After Surgery in the Danish Realm,” Regional Anesthesia and Pain Medicine 46, no. 11 (2021): 948–953. - PubMed
-
- Katz J. and Seltzer Z., “Transition From Acute to Chronic Postsurgical Pain: Risk Factors and Protective Fa,” Expert Review of Neurotherapeutics 9, no. 5 (2009): 723–744. - PubMed
-
- Kehlet H., Jensen T. S., and Woolf C. J., “Persistent Postsurgical Pain: Risk Factors and Prevention,” Lancet 367, no. 9522 (2006): 1618–1625. - PubMed
-
- Lavand'homme P., “Transition From Acute to Chronic Pain After Surgery,” Pain 158, no. Suppl 1 (2017): S50–S54. - PubMed
-
- Uhrbrand P., Helmig P., Haroutounian S., Vistisen S. T., and Nikolajsen L., “Persistent Opioid Use After Spine Surgery: A Prospective Cohort Study,” Spine 46, no. 20 (2021): 1428–1435. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

