Antiretroviral Postexposure Prophylaxis After Sexual, Injection Drug Use, or Other Nonoccupational Exposure to HIV - CDC Recommendations, United States, 2025
- PMID: 40331832
- PMCID: PMC12064164
- DOI: 10.15585/mmwr.rr7401a1
Antiretroviral Postexposure Prophylaxis After Sexual, Injection Drug Use, or Other Nonoccupational Exposure to HIV - CDC Recommendations, United States, 2025
Abstract
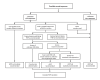
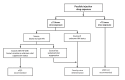
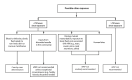
Nonoccupational postexposure prophylaxis (nPEP) for HIV is recommended when a nonoccupational (e.g., sexual, needle, or other) exposure to nonintact skin or mucous membranes that presents a substantial risk for HIV transmission has occurred, and the source has HIV without sustained viral suppression or their viral suppression information is not known. A rapid HIV test (also referred to as point-of-care) or laboratory-based antigen/antibody combination HIV test is recommended before nPEP initiation. Health care professionals should ensure the first dose of nPEP is provided as soon as possible, and ideally within 24 hours, but no later than 72 hours after exposure. The initial nPEP dose should not be delayed due to pending results of any laboratory-based testing, and the recommended length of nPEP course is 28 days. The recommendations in these guidelines update the 2016 nPEP guidelines (CDC. Updated guidelines for antiretroviral postexposure prophylaxis after sexual, injection drug use, or other nonoccupational exposure to HIV - United States, 2016. Atlanta, GA: US Department of Health and Human Services, CDC; 2017). These 2025 nPEP guidelines update recommendations and considerations for use of HIV nPEP in the United States to include newer antiretroviral (ARV) agents, updated nPEP indication considerations, and emerging nPEP implementation strategies. The guidelines also include considerations for testing and nPEP regimens for persons exposed who have received long-acting injectable ARVs in the past. Lastly, testing recommendations for persons who experienced sexual assault were updated to align with the most recent CDC sexually transmitted infection treatment guidelines. These guidelines are divided into two sections: Recommendations and CDC Guidance. The preferred regimens for most adults and adolescents are now bictegravir/emtricitabine/tenofovir alafenamide or dolutegravir plus (tenofovir alafenamide or tenofovir disoproxil fumarate) plus (emtricitabine or lamivudine). However, the regimen can be tailored to the clinical circumstances. Medical follow-up for persons prescribed nPEP also should be tailored to the clinical situation; recommended follow-up includes a visit at 24 hours (remote or in person) with a medical provider, and clinical follow-up 4-6 weeks and 12 weeks after exposure for laboratory testing. Persons initiating nPEP should be informed that pre-exposure prophylaxis for HIV (PrEP) can reduce their risk for acquiring HIV if they will have repeat or continuing exposure to HIV after the end of the nPEP course. Health care professionals should offer PrEP options to persons with ongoing indications for PrEP and create an nPEP-to-PrEP transition plan for persons who accept PrEP.
Conflict of interest statement
All authors have completed and submitted the International Committee of Medical Journal Editors form for the disclosure of any potential conflicts of interest. No potential conflicts of interest were reported.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
