Study of Cytotoxicity of 3-Azabicyclo[3.1.0]hexanes and Cyclopropa[ a]pyrrolizidines Spiro-Fused to Acenaphthylene-1(2 H)-one and Aceanthrylene-1(2 H)-one Fragments Against Tumor Cell Lines
- PMID: 40331956
- PMCID: PMC12026830
- DOI: 10.3390/ijms26083474
Study of Cytotoxicity of 3-Azabicyclo[3.1.0]hexanes and Cyclopropa[ a]pyrrolizidines Spiro-Fused to Acenaphthylene-1(2 H)-one and Aceanthrylene-1(2 H)-one Fragments Against Tumor Cell Lines
Abstract
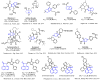
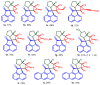
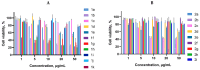
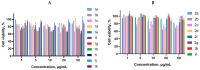
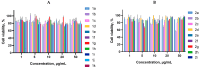
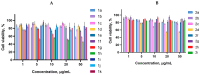
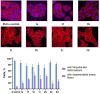
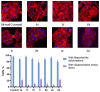
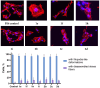
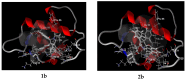
A series of 3-azabicyclo[3.1.0]hexanes and cyclopropa[a]pyrrolizidines spiro-fused to acenaphthylene-1(2H)-one and aceanthrylene-1(2H)-one frameworks have been studied for their in vitro antiproliferative activity against human erythroleukemia (K562), cervical carcinoma (HeLa), melanoma (Sk-mel-2), osteosarcoma (U2OS), as well as murine melanoma (B16) cell lines. Using confocal microscopy, it was found that cultivation with the tested spiro-fused compounds led to the disappearance of stress fibers (granular actin was distributed diffusely in the cytoplasm in up to 56% of treated cells) and decrease in filopodia-like deformations (up to 69% after cultivation), which indirectly suggests a decrease in cell motility. The human melanoma cell line scratch test showed that these cells lose their ability to move after cultivation with the tested spiro-fused compounds and do not fill the scratched strip. This was also supported by docking simulations with actin-related targets (PDB ID: 8DNH, 2Q1N). Using flow cytometry, the impact on the mitochondrial membrane potential showed that the tested compounds led to a significant increase in the number of cells with decreased mitochondrial membrane potential from 10% for the control up to 55-80% for the cyclopropa[a]pyrrolizidine adducts. The obtained results support the antitumor effect of the tested spiro-compounds and encourage the extension of the study in order to improve their anticancer activity as well as reduce their toxicological risks.
Keywords: 1,3-dipolar cycloaddition; 3-spiro[3-azabicyclo[3.1.0]-hexanes]; antiproliferative activity; azomethine ylides; cell motility; cyclopropa[a]pyrrolizidines; cyclopropenes; morphological changes (cytoskeleton); spiro-acenaphthylene-1,2′-bicyclo[3.1.0]hexanes; spiro-acenaphthylene-1,2′-cyclopropa[a]pyrrolizines; tumor cell lines.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

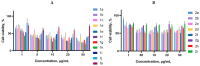




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

