The Prognostic Significance of Epidermal Growth Factor Receptor Amplification and Epidermal Growth Factor Receptor Variant III Mutation in Glioblastoma: A Systematic Review and Meta-Analysis with Implications for Targeted Therapy
- PMID: 40331985
- PMCID: PMC12027172
- DOI: 10.3390/ijms26083539
The Prognostic Significance of Epidermal Growth Factor Receptor Amplification and Epidermal Growth Factor Receptor Variant III Mutation in Glioblastoma: A Systematic Review and Meta-Analysis with Implications for Targeted Therapy
Abstract
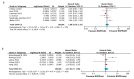
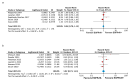
Glioblastoma (GBM) is the most aggressive and heterogeneous neoplasm among central nervous system tumors, with a dismal prognosis and a high recurrence rate. Among the various genetic alterations found in GBM, the amplification of epidermal growth factor receptor (EGFR) and the EGFR variant III (EGFRvIII) mutation are among the most common, though their prognostic value remains controversial. This systematic review and meta-analysis aimed to provide a comprehensive evaluation of the diagnostic and prognostic significance of EGFR amplification and the EGFRvIII mutation in GBM patients, incorporating recent studies published in the past few years to offer a more complete and up-to-date analysis. An extensive search of the PubMed, Web of Science, and Scopus databases was conducted, including studies that reported on EGFR and/or the EGFRvIII mutation status with detailed survival data. A total of 32 studies with 4208 GBM patients were included. The results indicated that EGFR amplification significantly correlated with worse OS (Overall survival) (HR = 1.27, 95% CI: 1.03-1.57), suggesting that EGFR amplification is an independent prognostic marker. The prognostic value of EGFRvIII was inconclusive, with a pooled hazard ratio for overall survival of 1.13 (95% CI: 0.94-1.36), indicating no significant effect on survival in the general population. However, a subgroup analysis suggested that EGFRvIII may be associated with poorer outcomes, particularly in recurrent GBM patients, where its prognostic significance became more evident. Furthermore, subgroup analyses based on geographic region revealed significant heterogeneity in the prognostic impact of EGFR amplification across different populations. In American cohorts, EGFR amplification was strongly associated with an increased risk of mortality (HR = 1.53, 95% CI: 1.28-1.84, p = 0.001), suggesting that it serves as a more reliable prognostic marker in this region. In contrast, no significant prognostic impact of EGFR amplification was observed in Asian (HR = 0.64, 95% CI: 0.35-1.17) or European (HR = 0.98, 95% CI: 0.80-1.19) populations. Overall, this study underscores the potential of EGFR amplification as a prognostic marker in GBM, while further research is needed to fully elucidate the role of the EGFRvIII mutation, particularly in specific patient subgroups. Clarifying these associations could offer important insights for targeted treatment strategies, improving patient outcomes.
Keywords: EGFR amplification; EGFRvIII mutation; glioblastoma (GBM); prognostic biomarker.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Snuderl M., Fazlollahi L., Le L.P., Nitta M., Zhelyazkova B.H., Davidson C.J., Akhavanfard S., Cahill D.P., Aldape K.D., Betensky R.A., et al. Mosaic amplification of multiple receptor tyrosine kinase genes in glioblastoma. Cancer Cell. 2011;20:810–817. doi: 10.1016/j.ccr.2011.11.005. - DOI - PubMed
-
- Mukherjee J., DeSouza L.V., Micallef J., Karim Z., Croul S., Siu K.W., Guha A. Loss of collapsin response mediator Protein1, as detected by iTRAQ analysis, promotes invasion of human gliomas expressing mutant EGFRvIII. Cancer Res. 2009;69:8545–8554. doi: 10.1158/0008-5472.CAN-09-1778. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

