Update on the Clinical and Molecular Characterization of Noonan Syndrome and Other RASopathies: A Retrospective Study and Systematic Review
- PMID: 40332000
- PMCID: PMC12027154
- DOI: 10.3390/ijms26083515
Update on the Clinical and Molecular Characterization of Noonan Syndrome and Other RASopathies: A Retrospective Study and Systematic Review
Abstract
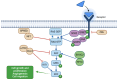
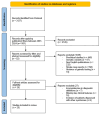
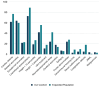
RASopathies are a diverse group of genetic conditions caused by hyperactivation of the RAS-MAPK signaling pathway, mainly inherited in an autosomal dominant manner. They present with variable features such as short stature, congenital heart defects, facial dysmorphisms, and neurodevelopmental delays. This study retrospectively analyzed 143 cases from 2003 to 2022, aiming to improve genotype-phenotype correlation knowledge for personalized care. Patients with genetically confirmed Noonan syndrome (NS) and related disorders were included, with molecular analysis performed via Sanger or parallel sequencing. Data from 906 previously reported cases were also reviewed. Among the 143 patients, most had NS (n = 116). PTPN11 mutations were most frequent (61%), followed by SOS1 (10.3%) and RAF1 (8.6%). Cardiac anomalies were observed in 71%, with pulmonary stenosis (PS) prevalent in NS (48.3%) and hypertrophic cardiomyopathy (HCM) in NSML (40%). PTPN11 variants were linked to PS and atrial septal defects, SOS1 to multiple cardiopathies, and RAF1 to HCM. Additional features included facial dysmorphisms (74.1%), short stature (62.0%), skeletal anomalies (43.1%), cryptorchidism (59.7%), and brain abnormalities (17.2%). JMML and other malignancies were seen in eight patients. This study emphasizes the importance of genotype-guided care, improved diagnosis of mild cases, and the underrecognized prevalence of neurological anomalies.
Keywords: PTPN11; RAF1; RAS/MAPK pathway; SOS1; congenital disorders.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Defining the variant-phenotype correlation in patients affected by Noonan syndrome with the RAF1:c.770C>T p.(Ser257Leu) variant.Eur J Hum Genet. 2024 Aug;32(8):964-971. doi: 10.1038/s41431-024-01643-6. Epub 2024 Jun 1. Eur J Hum Genet. 2024. PMID: 38824260 Free PMC article.
-
Clinical and molecular characterization of children with Noonan syndrome and other RASopathies in Argentina.Arch Argent Pediatr. 2019 Oct 1;117(5):330-337. doi: 10.5546/aap.2019.eng.330. Arch Argent Pediatr. 2019. PMID: 31560489 English, Spanish.
-
PTPN11, SOS1, KRAS, and RAF1 gene analysis, and genotype-phenotype correlation in Korean patients with Noonan syndrome.J Hum Genet. 2008;53(11-12):999-1006. doi: 10.1007/s10038-008-0343-6. Epub 2008 Nov 20. J Hum Genet. 2008. PMID: 19020799
-
Are RASopathies new monogenic predisposing conditions to the development of systemic lupus erythematosus? Case report and systematic review of the literature.Semin Arthritis Rheum. 2013 Oct;43(2):217-9. doi: 10.1016/j.semarthrit.2013.04.009. Epub 2013 Jun 17. Semin Arthritis Rheum. 2013. PMID: 23786871
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
Cited by
-
Complete commissural agenesis in a child with Noonan-like syndrome with loose anagen hair 2.Neurogenetics. 2025 Jun 30;26(1):53. doi: 10.1007/s10048-025-00832-9. Neurogenetics. 2025. PMID: 40586991
References
-
- Allanson J.E., Roberts A.E. Cassidy and Allanson’s Management of Genetic Syndromes. Wiley; Hoboken, NJ, USA: 2021. Noonan Syndrome; pp. 651–669.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

