Immune Response Against Recent Omicron Sub-Lineages in Persons with HIV Receiving a Protein-Based or mRNA XBB.1.5 SARS-CoV-2 Booster Vaccine
- PMID: 40332006
- PMCID: PMC12027319
- DOI: 10.3390/ijms26083521
Immune Response Against Recent Omicron Sub-Lineages in Persons with HIV Receiving a Protein-Based or mRNA XBB.1.5 SARS-CoV-2 Booster Vaccine
Abstract
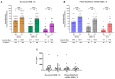
The new Nuvaxovid protein-based and Pfizer-BioNTech mRNA-based vaccines targeting Omicron XBB.1.5 were available during the 2023-2024 autumn/winter vaccination campaign for frail individuals, including people with HIV (PWH). We assessed the immune response in 51 PWH on stable ART who received a booster with either the Nuvaxovid protein-based (n = 25) or Pfizer-BioNTech mRNA-based XBB.1.5 vaccine (n = 26). The median age was 57 years (IQR 51-65), the median count of CD4 at T0 was 652/mmc (503-935), and CD4 nadir was 226/mmc (95-340). Samples were collected before (T0) and one month after (T1) the booster. We measured neutralizing antibodies (nAbs) titers against D614G, XBB.1.6, and JN.1 variants and T-cell IFN-γ levels produced upon specific stimulation. Regardless of the vaccine used, we observed a marked increase in nAbs titers from T0 to T1 against all the subvariants, but no evidence for a change in IFN-γ release. After controlling for confounders, there was no evidence for a difference in the T0-T1 change in nAbs titers against XBB.1.16 and JN.1 by the type of vaccine, while Nuvaxovid determined a smaller increase in D614G nAbs (p = 0.008). The XBB.1.5 protein-based vaccine's immunogenicity as a fifth or later booster was comparable to the Pfizer-BioNTech mRNA vaccine, particularly against recent Omicron variants.
Keywords: COVID-19; PWH; SARS-CoV-2 XBB.1.5 vaccine; immunogenicity; neutralizing response.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Allen H., Tessier E., Turner C., Anderson C., Blomquist P., Simons D., Løchen A., Jarvis C.I., Groves N., Capelastegui F., et al. Comparative transmission of SARS-CoV-2 Omicron (B.1.1.529) and Delta (B.1.617.2) variants and the impact of vaccination: National cohort study, England. Epidemiol. Infect. 2023;151:e58. doi: 10.1017/S0950268823000420. - DOI - PMC - PubMed
-
- Stefanelli P., Trentini F., Petrone D., Mammone A., Ambrosio L., Manica M., Guzzetta G., d’Andrea V., Marziano V., Zardini A., et al. Tracking the progressive spread of the SARS-CoV-2 Omicron variant in Italy, December 2021 to January 2022. Eurosurveillance. 2022;27:2200125. doi: 10.2807/1560-7917.ES.2022.27.45.2200125. - DOI - PMC - PubMed
-
- RCP Comirnaty Omicron XBB.1.5. [(accessed on 27 March 2025)]; Available online: https://www.aifa.gov.it/documents/20142/1279946/RCP_COMIRNATY_XBB.pdf.
-
- Circolare Ministeriale del 22/2/2022 Indicazione di Utilizzo del Vaccino Anti COVID19 Nuvaxovid (Novavax) nei Soggetti Di età Pari o Superiore ai 18 Anni. [(accessed on 27 March 2025)]; Available online: https://www.trovanorme.salute.gov.it/norme/renderNormsanPdf?anno=2022&co....
-
- Stankov M.V., Hoffmann M., Jauregui R.G., Cossmann A., Ramos G.M., Graalmann T., Winter E.J., Friedrichsen M., Ravens I., Ilievska T., et al. Humoral and cellular immune responses following BNT162b2 XBB.1.5 vaccination. Lancet Infect. Dis. 2024;24:e1–e3. doi: 10.1016/S1473-3099(23)00690-4. - DOI - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

