Extracellular Cold-Inducible RNA-Binding Protein: Progress from Discovery to Present
- PMID: 40332009
- PMCID: PMC12026706
- DOI: 10.3390/ijms26083524
Extracellular Cold-Inducible RNA-Binding Protein: Progress from Discovery to Present
Abstract
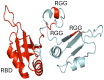
Extracellular cold-inducible RNA-binding protein (eCIRP) is a critical damage-associated molecular pattern (DAMP) that drives inflammation and tissue injury in hemorrhagic and septic shock, and has emerged as a promising therapeutic target. Since then, extensive research using preclinical models of diseases and patient materials has explored eCIRP's role in driving inflammatory responses and its potential as a biomarker. The main objective of this comprehensive review is to provide a detailed overview of eCIRP, covering its discovery, role in disease pathophysiology, mechanisms of release and action, potential as a biomarker, and therapeutic strategies targeting eCIRP in preclinical models of inflammatory and ischemic diseases. We examine the molecular, cellular, and immunological mechanisms through which eCIRP contributes to disease progression, and explore both well-established and emerging areas of research. Furthermore, we discuss potential therapeutic strategies targeting eCIRP across a broad spectrum of inflammatory conditions, including shock, ischemia-reperfusion injury, neurodegenerative diseases, and radiation injury.
Keywords: DAMPs; diseases; immune cells; immunity; inflammation.
Conflict of interest statement
M.A. and P.W. are inventors on a provisional patent application related to this manuscript [A Chimeric Compound for Simultaneously Targeting of Multiple Damage-Associated Molecular Patterns (DAMPs) for Treatment of Inflammatory Diseases. U.S. Provisional Application No.: 63/701,015, Filed on 30 September 2024]. The other author (I.H.C.) has no conflicts of interest.
Figures
Similar articles
-
Extracellular CIRP (eCIRP) and inflammation.J Leukoc Biol. 2019 Jul;106(1):133-146. doi: 10.1002/JLB.3MIR1118-443R. Epub 2019 Jan 15. J Leukoc Biol. 2019. PMID: 30645013 Free PMC article. Review.
-
Extracellular CIRP as an endogenous TREM-1 ligand to fuel inflammation in sepsis.JCI Insight. 2020 Mar 12;5(5):e134172. doi: 10.1172/jci.insight.134172. JCI Insight. 2020. PMID: 32027618 Free PMC article.
-
The protective effect of H151, a novel STING inhibitor, in renal ischemia-reperfusion-induced acute kidney injury.Am J Physiol Renal Physiol. 2023 Jun 1;324(6):F558-F567. doi: 10.1152/ajprenal.00004.2023. Epub 2023 Apr 27. Am J Physiol Renal Physiol. 2023. PMID: 37102684 Free PMC article.
-
Extracellular CIRP Induces an Inflammatory Phenotype in Pulmonary Fibroblasts via TLR4.Front Immunol. 2021 Jul 23;12:721970. doi: 10.3389/fimmu.2021.721970. eCollection 2021. Front Immunol. 2021. PMID: 34367191 Free PMC article.
-
Anti-DAMP therapies for acute inflammation.Front Immunol. 2025 May 8;16:1579954. doi: 10.3389/fimmu.2025.1579954. eCollection 2025. Front Immunol. 2025. PMID: 40406124 Free PMC article. Review.
Cited by
-
Extracellular CIRP dysregulates microglial efferocytosis in ischemic stroke via the TLR4/miR-155/MafB axis.Res Sq [Preprint]. 2025 Jul 31:rs.3.rs-7223452. doi: 10.21203/rs.3.rs-7223452/v1. Res Sq. 2025. PMID: 40766256 Free PMC article. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

