Lactobacillus helveticus HY7804 Modulates the Gut-Liver Axis to Improve Metabolic Dysfunction-Associated Steatotic Liver Disease in a Mouse Model
- PMID: 40332012
- PMCID: PMC12027198
- DOI: 10.3390/ijms26083557
Lactobacillus helveticus HY7804 Modulates the Gut-Liver Axis to Improve Metabolic Dysfunction-Associated Steatotic Liver Disease in a Mouse Model
Abstract
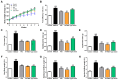
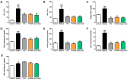
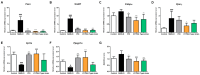
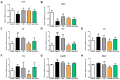
Metabolic dysfunction-associated steatotic liver disease (MASLD) is the most common type of liver disease worldwide. In a previous study, we confirmed that Lactobacillus helveticus HY7804 (HY7804) improves MASLD by suppressing the expression of mRNAs encoding genes related to hepatic lipogenesis, inflammation, and fibrosis in model mice. Here, we evaluated the ability of HY7804 to restore intestinal barrier function and modulate the gut microbiota, as well as improve MASLD symptoms. Mice fed an MASLD-inducing diet for 7 weeks received HY7804 (109 CFU/kg/day), the Type strain, or positive control (Pioglitazone) during the same period. HY7804 alleviated physiological (p < 0.001) and blood biochemical indicators and reduced MASLD activity scores (p < 0.05) on histological analysis. In addition, HY7804 increased the expression of genes related to fatty acid oxidation (p < 0.001); decreased the expression of apoptosis-related genes (p < 0.001); rescued the expression of tight junction (TJ)-related genes (p < 0.05); and suppressed the expression of pro-inflammatory cytokines and TLR4/MyD88/NF-κB signaling (p < 0.01) in the intestine. Finally, HY7804 modulated the composition of the gut microbiota in MASLD-induced mice. HY7804 increased the abundance of MASLD-suppressive Bacteroidaceae and Bacteroides, which positively correlated with the expression of TJ- and fatty acid oxidation-related genes. By contrast, HY7804 decreased the abundance of bacteria related to the progression of MASLD, including Cloastridaceae, Clostridium, Streptococcaceae, Lactococcus, and Lachnospiraceae, which correlated with intestinal immune responses and MASLD symptoms. In conclusion, L. helveticus HY7804 may be suitable as a functional supplement that alleviates MASLD symptoms and improves intestinal health.
Keywords: Lactobacillus helveticus; MASLD; gut microbiota; intestinal inflammation; tight junction.
Conflict of interest statement
All Authors were employed by the company hy Co., Ltd. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Oryzanol ameliorates MCD-induced metabolic dysfunction-associated steatohepatitis in mice via gut microbiota reprogramming and TLR4/NF-κB signaling suppression.Am J Physiol Gastrointest Liver Physiol. 2025 May 1;328(5):G578-G593. doi: 10.1152/ajpgi.00190.2024. Epub 2025 Apr 17. Am J Physiol Gastrointest Liver Physiol. 2025. PMID: 40243180
-
Alpha-aminobutyric acid ameliorates diet-induced metabolic dysfunction-associated steatotic liver disease (MASLD) progression in mice via enhancing AMPK/SIRT1 pathway and modulating the gut-liver axis.J Nutr Biochem. 2025 Jun;140:109885. doi: 10.1016/j.jnutbio.2025.109885. Epub 2025 Feb 25. J Nutr Biochem. 2025. PMID: 40015656
-
Lactobacillus helveticus R0052 alleviates liver injury by modulating gut microbiome and metabolome in D-galactosamine-treated rats.Appl Microbiol Biotechnol. 2019 Dec;103(23-24):9673-9686. doi: 10.1007/s00253-019-10211-8. Epub 2019 Nov 12. Appl Microbiol Biotechnol. 2019. PMID: 31713675
-
Advances in the acting mechanism and treatment of gut microbiota in metabolic dysfunction-associated steatotic liver disease.Gut Microbes. 2025 Dec;17(1):2500099. doi: 10.1080/19490976.2025.2500099. Epub 2025 May 20. Gut Microbes. 2025. PMID: 40394806 Free PMC article. Review.
-
Microbiome-centered therapies for the management of metabolic dysfunction-associated steatotic liver disease.Clin Mol Hepatol. 2025 Feb;31(Suppl):S94-S111. doi: 10.3350/cmh.2024.0811. Epub 2024 Nov 28. Clin Mol Hepatol. 2025. PMID: 39604327 Free PMC article. Review.
References
-
- Quek J., Chan K.E., Wong Z.Y., Tan C., Tan B., Lim W.H., Tan D.J.H., Tang A.S.P., Tay P., Xiao J. Global prevalence of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in the overweight and obese population: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2023;8:20–30. doi: 10.1016/S2468-1253(22)00317-X. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

