Environmental Factors Exacerbate Parkinsonian Phenotypes in an Asian-Specific Knock-In LRRK2 Risk Variant in Mice
- PMID: 40332013
- PMCID: PMC12027425
- DOI: 10.3390/ijms26083556
Environmental Factors Exacerbate Parkinsonian Phenotypes in an Asian-Specific Knock-In LRRK2 Risk Variant in Mice
Abstract
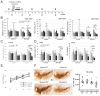
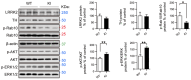
Parkinson's disease (PD) is a neurodegenerative disorder affecting nearly 10 million people worldwide, and for which no cure is currently known. Mutations in the Leucine-Rich Repeat Kinase 2 (LRRK2) gene, age, as well as environmental factors such as neurotoxin exposure and stress, are known to increase the risk of developing the disease in humans. To investigate the role of a specific Asian variant of the LRRK2 gene to induce susceptibility to stress and trigger PD phenotypes with time, knock-in (KI) mice bearing the human LRRK2 R1628P risk variant have been generated and studied from 2 to 16 months of age in the presence (or absence) of stress insults, including neurotoxin injections and chronic mild stress applied at 3 months of age. Pathophysiological and behavioural phenotypes have been measured at different ages and primary neurons and fibroblast cells were cultured from the KI mouse line and treated with H2O2 to study susceptibility towards oxidative stress in vitro. KI mice displayed specific PD features and these phenotypes were aggravated by environmental stresses. In particular, KI mice developed locomotion impairment and increased constipation. In addition, dopamine-related proteins were dysregulated in KI mice brains: Dopamine transporter (DAT) was decreased in the midbrain and striatum and dopamine levels were increased. Primary fibroblast cells and cortical neurons from KI mice also displayed increased susceptibility to oxidative stress. Therefore, the LRRK2 R1628P KI mice are an excellent model to study the progressive development of PD.
Keywords: Asian specific variant; LRRK2; Parkinson’s disease; behaviour; environmental toxins; oxidative stress; physiological stress.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

