From Skeletal Muscle to Myocardium: Molecular Mechanisms of Exercise-Induced Irisin Regulation of Cardiac Fibrosis
- PMID: 40332022
- PMCID: PMC12026460
- DOI: 10.3390/ijms26083550
From Skeletal Muscle to Myocardium: Molecular Mechanisms of Exercise-Induced Irisin Regulation of Cardiac Fibrosis
Abstract
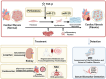
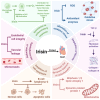
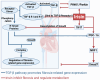
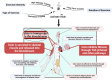
This study systematically elucidates the regulatory mechanisms and potential therapeutic value of the exercise-induced hormone Irisin in the pathological progression of cardiac fibrosis. Through comprehensive analysis and multidimensional data integration, we constructed a complete regulatory network of Irisin within the cardiovascular system, spanning its secretion, signal transduction, and precise regulatory control. Our findings demonstrate that exercise intervention significantly elevates circulating Irisin levels via the skeletal muscle-peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α)-fibronectin type III domain-containing protein 5 (FNDC5) signaling axis. Irisin establishes a multidimensional molecular barrier against cardiac fibrosis by targeting Sirtuin 1 (Sirt1) activation, inhibiting the transforming growth factor-beta (TGF-β)/Smad3 signaling pathway, and modulating the transcriptional activity of the mitochondrial biogenesis core factors PGC-1α and nuclear respiratory factor 1 (NRF-1). Moreover, the dual regulatory mechanism of the exercise-skeletal muscle-heart axis not only effectively suppresses the aberrant activation of cardiac fibroblasts but also significantly reduces collagen deposition, oxidative stress, and inflammatory infiltration by restoring mitochondrial dynamics balance. Taken together, this study reveals a novel exercise-mediated cardioprotective mechanism at the molecular interaction network level, thereby providing a theoretical basis for the development of non-pharmacological bio-intervention strategies targeting the Irisin signaling pathway and laying a translational foundation for precise exercise prescriptions in cardiovascular diseases.
Keywords: Irisin; cardiac fibrosis; cardioprotection; exercise.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Naghavi M., Abajobir A.A., Abbafati C., Abbas K.M., Abd-Allah F., Abera S.F., Aboyans V., Adetokunboh O., Afshin A., Agrawal A., et al. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1151–1210. doi: 10.1016/S0140-6736(17)32152-9. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

