Role of Thalamic CaV3.1 T-Channels in Fear Conditioning
- PMID: 40332044
- PMCID: PMC12026627
- DOI: 10.3390/ijms26083543
Role of Thalamic CaV3.1 T-Channels in Fear Conditioning
Abstract
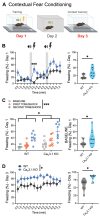
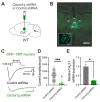
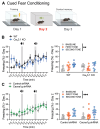
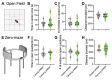
The potential contribution of the ion channels that control the excitability of the midline and intralaminar nuclei of the thalamus to the modulation of behaviors has not been well studied. In this study, we used both global genetic deletion (knock-out, KO) and thalamus-specific molecular knock-down (KD) approaches to investigate the role of thalamic CaV3.1 T-type calcium channels (T-channels) in fear learning and fear responses. Previously, we have shown that the dominant subtype of T-channels in the central medial nucleus of the thalamus (CMT) is the CaV3.1 isoform and that CMT neurons from CaV3.1 KO animals have decreased burst firing. By specifically knocking down CaV3.1 T-channels in the CMT using the shRNA approach, we also reduced burst firing without affecting the tonic firing mode of the transfected neurons. We report that global CaV3.1 KO animals showed stronger freezing behaviors during both the conditioning and testing phases of contextual fear conditioning, while CMT-specific CaV3.1 KD mice only had stronger fear responses during testing. In contrast, the cue-mediated fear responses were similar between CaV3.1 KO and CaV3.1 KD mice and the controls. Our findings validate thalamic CaV3.1 T-channels as a potential new target for the development or treatment of different psychiatric diseases, such as post-traumatic stress disorder, schizophrenia, anxiety, and substance abuse disorders.
Keywords: T-type calcium channels; central medial nucleus of thalamus; fear conditioning.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Fanselow M.S., Sterlace S.R. The Wiley Blackwell Handbook of Operant and Classical Conditioning. Wiley; Hoboken, NJ, USA: 2014. Pavlovian Fear Conditioning; pp. 117–141. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

