α2-Adrenergic Receptors in Hypothalamic Dopaminergic Neurons: Impact on Food Intake and Energy Expenditure
- PMID: 40332078
- PMCID: PMC12026701
- DOI: 10.3390/ijms26083590
α2-Adrenergic Receptors in Hypothalamic Dopaminergic Neurons: Impact on Food Intake and Energy Expenditure
Abstract
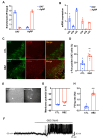
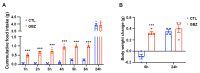
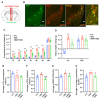
The adrenergic system plays an active role in modulating synaptic transmission in hypothalamic neurocircuitry. While α2-adrenergic receptors are widely distributed in various organs and are involved in various physiological functions, their specific role in the regulation of energy metabolism in the brain remains incompletely understood. Herein, we investigated the functions of α2-adrenergic receptors in the hypothalamus on energy metabolism in mice. Our study confirmed the expression of α2-adrenergic receptors in hypothalamic dopaminergic neurons and assessed metabolic phenotypes, including food intake and energy expenditure, after treatment with guanabenz, an α2-adrenergic receptor agonist. Guanabenz treatment significantly increased food intake (0.25 ± 0.03 g vs. 0.98 ± 0.05 g, p < 0.001) and body weight (-0.1 ± 0.04 g vs. 0.33 ± 0.03 g, p < 0.001) within 6 h post-treatment. Furthermore, guanabenz markedly elevated energy expenditure parameters, including respiratory exchange ratio (RER, 1.017 ± 0.007 vs. 1.113 ± 0.03, p < 0.01) and carbon dioxide production (1.512 ± 0.018 mL/min vs. 1.635 ± 0.036 mL/min, p < 0.05), compared to vehicle-treated controls. Furthermore, using chemogenetic techniques, we demonstrated that the altered metabolic phenotypes induced by guanabenz treatment were effectively reversed by inhibiting the activity of dopaminergic neurons in the hypothalamic arcuate nucleus (ARC) using a chemogenetic technique. Our findings suggest functional connectivity between hypothalamic α2-adrenergic receptor signals and dopaminergic neurons in metabolic controls.
Keywords: alpha-2 adrenergic receptor; energy expenditure; food intake; hypothalamic dopamine neurons.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

