LONP1 facilitates pulmonary artery smooth muscle cell glycolytic reprogramming by degrading MPC1 in pulmonary hypertension
- PMID: 40332105
- PMCID: PMC12203999
- DOI: 10.1042/CS20255922
LONP1 facilitates pulmonary artery smooth muscle cell glycolytic reprogramming by degrading MPC1 in pulmonary hypertension
Abstract
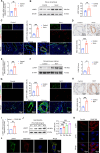
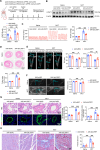
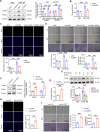
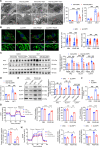
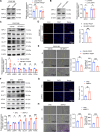
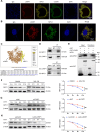
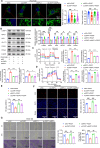
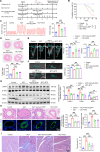
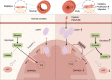
Pulmonary hypertension (PH) is a chronic and life-threatening disease characterized by pulmonary vascular remodeling (PVR), which involves the abnormal proliferation of pulmonary artery smooth muscle cells (PASMCs). These cells exhibit metabolic characteristics akin to cancer cells, particularly in their shift toward glycolysis. The Lon protease 1 (LONP1) has been shown to promote glycolytic reprogramming of tumor cells, conferring a malignant proliferative phenotype. However, the precise role of LONP1 in PH remains unclear. In the present study, Su5416/hypoxia-induced and monocrotaline (MCT)-induced PH rodent models and platelet-derived growth factor BB (PDGF-BB)-induced PASMCs were used to investigate the role and mechanism of LONP1 in PH. The results revealed an up-regulation of LONP1 expression in lung tissues from two PH rodent models, as well as in PDGF-BB-induced PASMCs. In vivo knockdown of LONP1 significantly alleviated PASMC mitochondrial dysfunction, reduced glycolytic enzyme expression, and decreased lactate accumulation, thereby mitigating PVR. Additionally, in vitro experiments demonstrated that knockdown or inhibition of LONP1 attenuated glycolytic reprogramming, proliferation, and migration of PASMCs, whereas overexpression of LONP1 had converse effects. Mechanistic studies confirmed that mitochondrial pyruvate carrier 1 (MPC1) was a direct substrate for LONP1-mediated degradation. Functional experiments with MPC1 knockdown and overexpression further elucidated its role in the proliferation and migration of PASMCs. Rescue experiments indicated that MPC1 knockdown abrogated the suppressive effects of LONP1 knockdown on glycolytic reprogramming, proliferation, and migration in PASMCs. Therapeutically, knockdown or pharmacological inhibition of LONP1 significantly reversed MCT-induced PH in rats. Thus, targeting LONP1 may represent a promising therapeutic strategy for PH.
Keywords: Lon protease 1; glycolytic reprogramming; mitochondrial pyruvate carrier 1; pulmonary hypertension; pulmonary vascular remodeling.
© 2025 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures
Similar articles
-
Novel and Highly Potent Therapeutic Agent, Trametes Robiniophila Murr (Huaier), Mitigates Pulmonary Vascular Remodeling in Rodents.J Am Heart Assoc. 2025 Aug 19;14(16):e041405. doi: 10.1161/JAHA.125.041405. Epub 2025 Aug 6. J Am Heart Assoc. 2025. PMID: 40767286
-
C-C Motif chemokine receptor-2 blockade ameliorates pulmonary hypertension in rats and synergizes with a pulmonary vasodilator.Cardiovasc Res. 2025 Jul 8;121(7):1076-1090. doi: 10.1093/cvr/cvae244. Cardiovasc Res. 2025. PMID: 39556088 Free PMC article.
-
5-Aminoimidazole-4-carboxamide ribonucleotide formyltransferase/inosine monophosphate cyclohydrolase promotes pulmonary arterial smooth muscle cell proliferation via the Ras signaling pathway.Am J Physiol Cell Physiol. 2024 Oct 1;327(4):C901-C912. doi: 10.1152/ajpcell.00262.2024. Epub 2024 Aug 12. Am J Physiol Cell Physiol. 2024. PMID: 39129491
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

