Synergistic HDAC4/8 Inhibition Sensitizes Osteosarcoma to Doxorubicin via pAKT/RUNX2 Pathway Modulation
- PMID: 40332124
- PMCID: PMC12026469
- DOI: 10.3390/ijms26083574
Synergistic HDAC4/8 Inhibition Sensitizes Osteosarcoma to Doxorubicin via pAKT/RUNX2 Pathway Modulation
Abstract
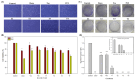
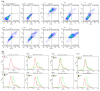
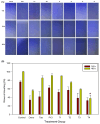
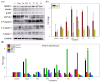
Osteosarcoma is a highly aggressive bone malignancy, particularly challenging in metastatic cases, with a 5-year survival rate remaining under 30%. Although doxorubicin (doxo) is a standard first-line chemotherapeutic agent, its clinical utility is often hindered by the development of drug resistance and associated systemic toxicity. Emerging evidence highlights the role of epigenetic alterations, particularly those involving histone deacetylases (HDACs), in promoting chemoresistance. In this context, the present study aimed to evaluate the therapeutic potential of combining doxo with the selective HDAC inhibitors, tasquinimod (Tas, targeting HDAC4) and PCI-34051 (PCI, targeting HDAC8), in SJSA-1 osteosarcoma cells. Utilizing both 2D and 3D in vitro models, the combination treatment (referred to as the T4 group) significantly reduced cell viability by 57.69% in 2D cultures and decreased spheroid volume by 35.19% in 3D models. The apoptotic response was markedly enhanced, with late apoptosis reaching 64.59% and necrosis at 32.07%, both surpassing the effects observed with doxo alone. Furthermore, wound healing assays demonstrated a 37.74% inhibition of migration, accompanied by a decreased expression of the matrix metalloproteinases MMP9 and MMP13. Mechanistically, the combination therapy led to the downregulation of protein kinase B (pAKT) and RUNX2, along with upregulation of apoptotic markers, including caspase 8, caspase 3, and cleaved caspase 3, indicating a disruption of key survival pathways. These findings suggest that dual HDAC inhibition with Tas and PCI can potentiate doxo efficacy by enhancing apoptosis, inhibiting proliferation, and reducing metastatic potential, thus offering a promising strategy to overcome chemoresistance in osteosarcoma. Further preclinical and clinical studies are required to validate these therapeutic benefits.
Keywords: HDAC inhibitor; anti-cancer; apoptosis; combination therapy; doxorubicin; osteosarcoma.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Sensitivity of osteosarcoma cells to HDAC inhibitor AR-42 mediated apoptosis.BMC Cancer. 2017 Jan 21;17(1):67. doi: 10.1186/s12885-017-3046-6. BMC Cancer. 2017. PMID: 28109246 Free PMC article.
-
CXCR4 blockade sensitizes osteosarcoma to doxorubicin by inducing autophagic cell death via PI3K‑Akt‑mTOR pathway inhibition.Int J Oncol. 2021 Jul;59(1):49. doi: 10.3892/ijo.2021.5229. Epub 2021 Jun 3. Int J Oncol. 2021. PMID: 34080667 Free PMC article.
-
Metformin synergistically enhances antitumor activity of histone deacetylase inhibitor trichostatin a against osteosarcoma cell line.DNA Cell Biol. 2013 Apr;32(4):156-64. doi: 10.1089/dna.2012.1926. Epub 2013 Mar 1. DNA Cell Biol. 2013. PMID: 23451817
-
Doxorubicin Inhibits Proliferation of Osteosarcoma Cells Through Upregulation of the Notch Signaling Pathway.Oncol Res. 2014;22(4):185-191. doi: 10.3727/096504015X14343704124340. Oncol Res. 2014. PMID: 26351207 Free PMC article.
-
Oleanane triterpenoid CDDO-Me induces apoptosis in multidrug resistant osteosarcoma cells through inhibition of Stat3 pathway.BMC Cancer. 2010 May 10;10:187. doi: 10.1186/1471-2407-10-187. BMC Cancer. 2010. PMID: 20459702 Free PMC article.
References
-
- Jing D., Wu W., Chen X., Xiao H., Zhang Z., Chen F., Zhang Z., Liu J., Shao Z., Pu F. Quercetin encapsulated in folic acid-modified liposomes is therapeutic against osteosarcoma by non-covalent binding to the JH2 domain of JAK2 via the JAK2-STAT3-PDL1. Pharmacol. Res. 2022;182:106287. doi: 10.1016/j.phrs.2022.106287. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

