Claudin-17 Deficiency Drives Vascular Permeability and Inflammation Causing Lung Injury
- PMID: 40332125
- PMCID: PMC12027279
- DOI: 10.3390/ijms26083612
Claudin-17 Deficiency Drives Vascular Permeability and Inflammation Causing Lung Injury
Abstract
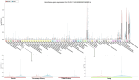
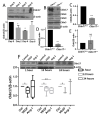
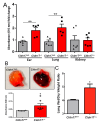
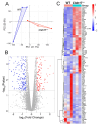
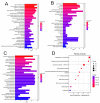
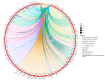
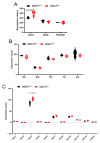
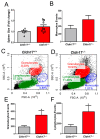
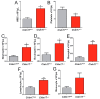
The role of claudin-17 (Cldn17), a tight-junction protein, in vascular permeability remains unclear. We investigated the impact of Cldn17 suppression on vascular permeability. The Miles assay demonstrated significantly increased vascular permeability in the lungs and skin of Cldn17-/- mice, as evidenced by elevated Evan's blue dye extravasation. The Matrigel plug assay demonstrated increased hemoglobin extravasation. Histopathological analysis revealed alveolar flooding, inflammatory cell infiltration, and lung injury in Cldn17-/- lungs. Wet/dry lung weight ratios indicated pulmonary edema, supporting the role of Cldn17 in pulmonary fluid balance, which was exacerbated with lipopolysaccharide administration. Ribosomal nucleic acid sequencing identified distinct transcriptional changes, with the principal component analysis showing clear clustering. Differential gene expression analysis highlighted significant alterations in inflammatory and metabolic pathways. Gene ontology and pathway enrichment analyses revealed the upregulation of immune-related processes, including leukocyte adhesion, interferon-gamma response, and neutrophil degranulation, alongside metabolic dysregulation affecting lipid transport and cytoskeletal organization. Reactome pathway analysis implicated Cldn17 in antigen presentation, interleukin-17 signaling, and inflammatory responses. These findings establish Cldn17 as a critical regulator of vascular permeability and immune homeostasis. Its deficiency drives vascular leakage, exacerbates lung injury, and alters immune signaling pathways, underscoring its potential as a therapeutic target for inflammatory lung diseases.
Keywords: Cldn17; inflammation; lung injury; pulmonary edema; vascular permeability.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

