Segatella copri Outer-Membrane Vesicles Are Internalized by Human Macrophages and Promote a Pro-Inflammatory Profile
- PMID: 40332148
- PMCID: PMC12027123
- DOI: 10.3390/ijms26083630
Segatella copri Outer-Membrane Vesicles Are Internalized by Human Macrophages and Promote a Pro-Inflammatory Profile
Abstract
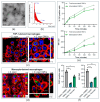
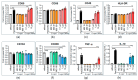
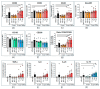
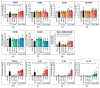
Increased abundance of Segatella copri (S. copri) within the gut microbiota is associated with systemic inflammatory diseases, including rheumatoid arthritis. Although outer-membrane vesicles (OMVs) of Gram-negative bacteria are important players in microbiota-host communication, the effect of S. copri-derived OMVs on immune cells is unknown. Macrophages engulf and eliminate foreign material and are conditioned by environmental signals to promote either homeostasis or inflammation. Thus, we aimed to explore the impact of S. copri-OMVs on human macrophages in vitro, employing THP-1 and monocyte-derived macrophage models. The uptake of DiO-labeled S. copri-OMVs into macrophages was monitored by confocal microscopy and flow cytometry. Furthermore, the effect of S. copri and S. copri-OMVs on the phenotype and cytokine secretion of naïve (M0), pro-inflammatory (M1), and anti-inflammatory (M2) macrophages was analyzed by flow cytometry and ELISA, respectively. We show that S. copri-OMVs enter human macrophages through macropinocytosis and clathrin-dependent mechanisms. S. copri-OMVs, but not the parental bacterium, induced a dose-dependent increase in the expression of M1-related surface markers in M0 and M2 macrophages and activated the secretion of large amounts of pro-inflammatory cytokines in M1 macrophages. These results highlight an important role of S. copri-OMVs in promoting pro-inflammatory macrophage responses, which might contribute to systemic inflammatory diseases.
Keywords: M1/M2 polarization; Segatella copri; endocytosis; macrophages; outer-membrane vesicles.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

