Crosstalk Between Sickle Cell Disease and Ferroptosis
- PMID: 40332185
- PMCID: PMC12027360
- DOI: 10.3390/ijms26083675
Crosstalk Between Sickle Cell Disease and Ferroptosis
Abstract
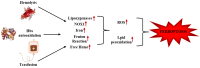
Sickle cell disease (SCD) is an inherited hemoglobin disorder that is widespread across the globe. It is characterized by a very complex pathogenesis, but at the basis of the disease is the mutation of the HBB gene, which determines the production of a mutated hemoglobin: sickle cell hemoglobin (HbS). The polymerization of HbS, which occurs when the protein is in a deoxygenated state, and the greater fragility of sickle cell red blood cells (sRBCs) determine the release of iron, free heme, and HbS in the blood, favoring oxidative stress and the production of reactive oxygen species (ROS). These features are common to the features of a new model of cell death known as ferroptosis, which is characterized by the increase of iron and ROS concentrations and by the inhibition of glutathione peroxidase 4 (GPx4) and the System Xc-. In this context, this review aims to discuss the potential molecular and biochemical pathways of ferroptosis involved in SCD, aiming to highlight possible tags involved in treating the disease and inhibiting ferroptosis.
Keywords: GPx4; cell death; iron overload; oxidative stress; sickle cell hemoglobin; system Xc−; transfusion.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

