Enzymes Drive Glutathione Shunt to Explain Oxidative State Using an In-Parallel Multi-Omic Method
- PMID: 40332189
- PMCID: PMC12026767
- DOI: 10.3390/ijms26083632
Enzymes Drive Glutathione Shunt to Explain Oxidative State Using an In-Parallel Multi-Omic Method
Abstract
The glutathione shunt is one of the most important contributors to the cellular redox state, with implications across cancer, chronic diseases, diseases of ageing, and autoimmune diseases, including inflammatory bowel disease (IBD). Traditionally, the redox state is gauged by the ratio of the surrogate metabolites GSH and GSSG. However, this presents methodological challenges and offers a constrained illustration of metabolites without a systems-level understanding of redox dynamics, failing to elucidate variations across an entire biochemical network. Targeted proteomics can fill this void. Here, we describe an in-parallel metabolomic and proteomic targeted method to encompass measurements directly related to the shunt. Samples are simultaneously prepared to extract the substrate building blocks, cysteine, cystine, methionine, glutamic acid, and kynurenine; and the proteins, SLC7A11 (xCT), Glutamate Cysteine Ligase (GSH1), Glutathione Synthetase (GSH2), Glutathione Peroxidase (GPx), and Glutathione Reductase (GSHR) for targeted mass spectrometry. We demonstrate the method by targeted analysis of proteins in plasma, serum, nasal swab, and saliva and apply the multi-omic method to assess changes in the glutathione shunt in the serum of patients diagnosed with IBD. This allows for a broader narrative to establish context at which the glutathione shunt is operating.
Keywords: GPx; GSH; GSSG; IBD; SLCA7A1; biomarker; glutathione shunt; liquid biopsy.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
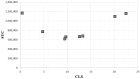







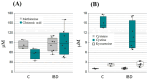
References
-
- Bustamante S., Yau Y., Boys V., Chang J., Paramsothy S., Pudipeddi A., Leong R.W., Wasinger V.C. Tryptophan Metabolism ‘Hub’ Gene Expression Associates with Increased Inflammation and Severe Disease Outcomes in COVID-19 Infection and Inflammatory Bowel Disease. Int. J. Mol. Sci. 2022;23:14776. doi: 10.3390/ijms232314776. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

