Uncovering a Novel Pathogenic Mechanism of BCS1L in Mitochondrial Disorders: Insights from Functional Studies on the c.38A>G Variant
- PMID: 40332224
- PMCID: PMC12027322
- DOI: 10.3390/ijms26083670
Uncovering a Novel Pathogenic Mechanism of BCS1L in Mitochondrial Disorders: Insights from Functional Studies on the c.38A>G Variant
Abstract
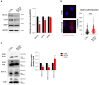
The BCS1L gene encodes a mitochondrial chaperone which inserts the Fe2S2 iron-sulfur Rieske protein into the nascent electron transfer complex III. Variants in the BCS1L gene are associated with a spectrum of mitochondrial disorders, ranging from mild to severe phenotypes. Björnstad syndrome, a milder condition, is characterized by sensorineural hearing loss (SNHL) and pili torti. More severe disorders include Complex III Deficiency, which leads to neuromuscular and metabolic dysfunctions with multi-systemic issues and Growth Retardation, Aminoaciduria, Cholestasis, Iron Overload, and Lactic Acidosis syndrome (GRACILE). The severity of these conditions varies depending on the specific BCS1L mutation and its impact on mitochondrial function. This study describes a 27-month-old child with SNHL, proximal renal tubular acidosis, woolly hypopigmented hair, developmental delay, and metabolic alterations. Genetic analysis revealed a homozygous BCS1L variant (c.38A>G, p.Asn13Ser), previously reported in a patient with a more severe phenotype that, however, was not functionally characterized. In this work, functional studies in a yeast model and patient-derived fibroblasts demonstrated that the variant impairs mitochondrial respiration, complex III activity (CIII), and also alters mitochondrial morphology in affected fibroblasts. Interestingly, we unveil a new possible mechanism of pathogenicity for BCS1L mutant protein. Since the interaction between BCS1L and CIII is increased, this suggests the formation of a BCS1L-containing nonfunctional preCIII unable to load RISP protein and complete CIII assembly. These findings support the pathogenicity of the BCS1L c.38A>G variant, suggesting altered interaction between the mutant BCS1L and CIII.
Keywords: BCS1L; assembly chaperone; complex III; electron transfer chain; mitochondrial disorder.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- De Vivo D.C., DiMauro S. Mitochondrial Diseases. 6th ed. Elsevier Inc.; Amsterdam, The Netherlands: 2017.
-
- Morán M., Marín-Buera L., Gil-Borlado M.C., Rivera H., Blázquez A., Seneca S., Vázquez-López M., Arenas J., Martín M.A., Ugalde C. Cellular Pathophysiological Consequences of BCS1L Mutations in Mitochondrial Complex III Enzyme Deficiency. Hum. Mutat. 2010;31:930–941. doi: 10.1002/humu.21294. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

