Improved Synthesis of Effective 3-(Indolin-6-yl)-4-(N-pyrazole-sulfonamide)-1 H-pyrrolo[2,3- b]pyridine-Based Inhibitors of NADPH Oxidase 2
- PMID: 40332246
- PMCID: PMC12026636
- DOI: 10.3390/ijms26083647
Improved Synthesis of Effective 3-(Indolin-6-yl)-4-(N-pyrazole-sulfonamide)-1 H-pyrrolo[2,3- b]pyridine-Based Inhibitors of NADPH Oxidase 2
Abstract
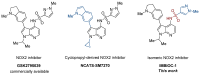
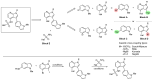
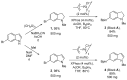
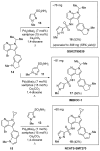
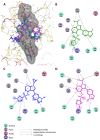
NADPH oxidase enzymes (NOXs) are a family of enzymes generating superoxide, which form reactive oxygen species. NOX2 activity is a causative agent for the progression of many diseases: neurodegenerative, cardiovascular, immune dysregulations, and even hereditary diseases and cancer. Administering antioxidants helps in inhibiting NOX2 activity; however, the development of selective inhibitors may provide greater improvement in the therapy of diseases. Here, an optimized synthesis of two most promising NOX2 inhibitors based on the 3-(indolin-6-yl)-4-(N-pyrazole-sulfonamide)-1H-pyrrolo [2,3-b]pyridine structure, namely, GSK2795039 and NCATS-SM7270, and an isomeric derivative of the same class, IMBIOC-1, is reported. The new modified procedures simplify the isolation, reduce byproduct formation, and improve the yields in 0.1-1 g scale preparations. Molecular modeling of the structures of NOX2 complexes with inhibitors validated their binding at the same site as NADPH, with IMBIOC-1 forming the largest number of intermolecular interactions with the NOX2 active site. Testing the effects of the compounds on amyloid beta-induced oxidative stress and toxicity in HMC3 microglial cells showed that all three inhibitors completely prevented the pathological amyloid-beta effect. At the same time, NCATS-SM7270 and IMBIOC-1 provided a stronger protective effect on microglial cell survival than GSK2795039, which allowed us to assert the potential of those compounds as neuroprotective agents.
Keywords: Alzheimer’s disease; GSK2795039; NOX2 inhibitor; microglial cells; reactive oxygen species.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
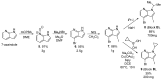
Similar articles
-
Discovery of GSK2795039, a Novel Small Molecule NADPH Oxidase 2 Inhibitor.Antioxid Redox Signal. 2015 Aug 10;23(5):358-74. doi: 10.1089/ars.2014.6202. Antioxid Redox Signal. 2015. PMID: 26135714 Free PMC article.
-
Development of an improved and specific inhibitor of NADPH oxidase 2 to treat traumatic brain injury.Redox Biol. 2023 Apr;60:102611. doi: 10.1016/j.redox.2023.102611. Epub 2023 Jan 18. Redox Biol. 2023. PMID: 36709665 Free PMC article.
-
Therapeutic potential of NADPH oxidase 1/4 inhibitors.Br J Pharmacol. 2017 Jun;174(12):1647-1669. doi: 10.1111/bph.13532. Epub 2016 Jul 14. Br J Pharmacol. 2017. PMID: 27273790 Free PMC article. Review.
-
NADPH oxidase 2 inhibitors CPP11G and CPP11H attenuate endothelial cell inflammation & vessel dysfunction and restore mouse hind-limb flow.Redox Biol. 2019 Apr;22:101143. doi: 10.1016/j.redox.2019.101143. Epub 2019 Feb 15. Redox Biol. 2019. PMID: 30897521 Free PMC article.
-
Targeting microglia-mediated neurotoxicity: the potential of NOX2 inhibitors.Cell Mol Life Sci. 2012 Jul;69(14):2409-27. doi: 10.1007/s00018-012-1015-4. Epub 2012 May 13. Cell Mol Life Sci. 2012. PMID: 22581365 Free PMC article. Review.
Cited by
-
Short-Term Inhibition of NOX2 Prevents the Development of Aβ-Induced Pathology in Mice.Antioxidants (Basel). 2025 May 30;14(6):663. doi: 10.3390/antiox14060663. Antioxidants (Basel). 2025. PMID: 40563297 Free PMC article.
References
-
- Mason H., Rai G., Kozyr A., De Jonge N., Gliniewicz E., Berg L.J., Wald G., Dorrier C., Henderson M.J., Zakharov A., et al. Development of an improved and specific inhibitor of NADPH oxidase 2 to treat traumatic brain injury. Redox Biol. 2023;60:102611. doi: 10.1016/j.redox.2023.102611. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

