Impact of Alterations in Homocysteine, Asymmetric Dimethylarginine and Vitamins-Related Pathways in Some Neurodegenerative Diseases: A Narrative Review
- PMID: 40332285
- PMCID: PMC12027465
- DOI: 10.3390/ijms26083672
Impact of Alterations in Homocysteine, Asymmetric Dimethylarginine and Vitamins-Related Pathways in Some Neurodegenerative Diseases: A Narrative Review
Abstract
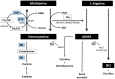
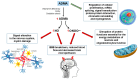
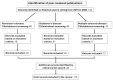
Hyperhomocysteinemia (HHcy) influences the development and progression of neurodegenerative disorders in different ways. Homocysteine (Hcy) metabolism is related to that of asymmetric dimethylarginine (ADMA) and group B vitamins. The breakdown of the pathway involving nitric oxide (NO) and ADMA can be considered one of the causes of endothelial alteration that represents a crucial step in the development of several neurodegenerative disorders. Deficiencies of vitamins other than group B ones, such as D and A, have also been associated with central nervous system disorders. The aim of this narrative review is to describe the link between HHcy, ADMA, and vitamins in Parkinson's disease (PD), Alzheimer's disease (AD), and multiple sclerosis (MS) in terms of dysfunctional pathways and neuropathological processes, performing a literature search from 2015 to 2025 on PubMed. This review also provides an overview of the effects of vitamin supplementation on neurodegenerative diseases. The alteration of pathways involving NO production can lead to HHcy and elevated ADMA concentrations, causing neurodegeneration through various mechanisms, while vitamin supplementation has been shown to reduce Hcy levels, although with conflicting results about the improvement in clinical symptoms. Further studies are needed to develop optimal combined therapeutic strategies.
Keywords: Alzheimer’s diseases; Parkinson’s disease; asymmetric dimethylarginine; hyperhomocysteinemia; multiple sclerosis; vitamins.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
[Homocysteine and asymmetric dimethylarginine (ADMA) in epilepsy].Przegl Lek. 2009;66(8):448-52. Przegl Lek. 2009. PMID: 20043592 Review. Polish.
-
Serum levels of homocysteine, asymmetric dimethylarginine and nitric oxide in patients with Parkinson's disease.Acta Clin Belg. 2016 Apr;71(2):71-5. doi: 10.1080/17843286.2016.1138592. Epub 2016 Feb 6. Acta Clin Belg. 2016. PMID: 27075796
-
ADMA and hyperhomocysteinemia.Vasc Med. 2005 Jul;10 Suppl 1:S27-33. doi: 10.1191/1358863x05vm599oa. Vasc Med. 2005. PMID: 16444866 Review.
-
[Review of the role of hyperhomocysteinemia and B-vitamin deficiency in neurological and psychiatric disorders--current evidence and preliminary recommendations].Fortschr Neurol Psychiatr. 2007 Sep;75(9):515-27. doi: 10.1055/s-2007-980112. Fortschr Neurol Psychiatr. 2007. PMID: 17729191 Review. German.
-
Concentrations of homocysteine, related metabolites and asymmetric dimethylarginine in preeclamptic women with poor nutritional status.Clin Chem Lab Med. 2005;43(10):1139-46. doi: 10.1515/CCLM.2005.198. Clin Chem Lab Med. 2005. PMID: 16197311
References
-
- Kirbas S., Kirbas A., Tufekci A., Cumhur Cure M., Cakmak S., Yazici T., Cure E. Serum Levels of Homocysteine, Asymmetric Dimethylarginine and Nitric Oxide in Patients with Parkinson’s Disease. Acta Clin. Belgica Int. J. Clin. Lab. Med. 2016;71:71–75. doi: 10.1080/17843286.2016.1138592. - DOI - PubMed
-
- Dayal S., Rodionov R.N., Arning E., Bottiglieri T., Kimoto M., Murry D.J., Cooke J.P., Faraci F.M., Lentz S.R. Tissue-Specific Downregulation of Dimethylarginine Dimethylaminohydrolase in Hyperhomocysteinemia. Am. J. Physiol.—Heart Circ. Physiol. 2008;295:816–825. doi: 10.1152/ajpheart.01348.2007. - DOI - PMC - PubMed
-
- Wang H., Huang Z.Q., Xia L., Feng Q., Erdjument-Bromage H., Strahl B.D., Briggs S.D., Allis C.D., Wong J., Tempst P., et al. Methylation of Histone H4 at Arginine 3 Facilitating Transcriptional Activation by Nuclear Hormone Receptor. Science. 2001;293:853–857. doi: 10.1126/science.1060781. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

