Paeoniflorin Directly Targets ENO1 to Inhibit M1 Polarization of Microglia/Macrophages and Ameliorates EAE Disease
- PMID: 40332313
- PMCID: PMC12027182
- DOI: 10.3390/ijms26083677
Paeoniflorin Directly Targets ENO1 to Inhibit M1 Polarization of Microglia/Macrophages and Ameliorates EAE Disease
Abstract
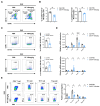
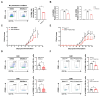
The chronic autoimmune disease multiple sclerosis (MS) now remains incurable. Paeoniflorin (PF), which is a monoterpene glucoside obtained from Paeonia lactiflora Pall, is recognized for neuroprotective and anti-inflammatory properties. However, the precise mechanism by which PF regulates MS is unclear. This work aims to elucidate the underlying mechanisms of PF in EAE, a well established animal model of MS, and to discover the target proteins that PF directly acts on. Our results revealed that PF administration can significantly attenuate the clinical symptoms of EAE and alleviate the central nervous system (CNS) inflammatory environment by inhibiting M1-type microglia/macrophages. Mechanistically, PF was found to directly interact with the glycolytic enzyme α-enolase (ENO1), inhibiting its enzymatic activity and expression to impair glucose metabolism, thereby suppressing microglia/macrophage M1 polarization and ameliorating CNS inflammation. Significantly, Eno1 knockdown in microglia/macrophages diminished their pro-inflammatory phenotype, while treatment with ENOBlock or the specific knockout of Eno1 in microglia led to EAE remission, underscoring the critical role of ENO1 in EAE progression. This study uncovers the molecular mechanism of PF in treating EAE, linking the anti-inflammatory property of PF to the glucose metabolism process, which will broaden the prospective applications of PF.
Keywords: EAE; ENO1; glucose metabolism; microglia/macrophage polarization; paeoniflorin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Wallin M.T., Culpepper W.J., Nichols E., Bhutta Z.A., Gebrehiwot T.T., Hay S.I., Khalil I.A., Krohn K.J., Liang X., Naghavi M., et al. Global, regional, and national burden of multiple sclerosis 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:269–285. doi: 10.1016/S1474-4422(18)30443-5. - DOI - PMC - PubMed
-
- Konen F.F., Mohn N., Witte T., Schefzyk M., Wiestler M., Lovric S., Hufendiek K., Schwenkenbecher P., Suhs K.W., Friese M.A., et al. Treatment of autoimmunity: The impact of disease-modifying therapies in multiple sclerosis and comorbid autoimmune disorders. Autoimmun. Rev. 2023;22:103312. doi: 10.1016/j.autrev.2023.103312. - DOI - PubMed
-
- Harding K., Williams O., Willis M., Hrastelj J., Rimmer A., Joseph F., Tomassini V., Wardle M., Pickersgill T., Robertson N., et al. Clinical Outcomes of Escalation vs Early Intensive Disease-Modifying Therapy in Patients with Multiple Sclerosis. JAMA Neurol. 2019;76:536–541. doi: 10.1001/jamaneurol.2018.4905. - DOI - PMC - PubMed
-
- Palace J., Duddy M., Bregenzer T., Lawton M., Zhu F., Boggild M., Piske B., Robertson N.P., Oger J., Tremlett H., et al. Effectiveness and cost-effectiveness of interferon beta and glatiramer acetate in the UK Multiple Sclerosis Risk Sharing Scheme at 6 years: A clinical cohort study with natural history comparator. Lancet Neurol. 2015;14:497–505. doi: 10.1016/S1474-4422(15)00018-6. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

