Drug Resistance: The Role of Sphingolipid Metabolism
- PMID: 40332322
- PMCID: PMC12027666
- DOI: 10.3390/ijms26083716
Drug Resistance: The Role of Sphingolipid Metabolism
Abstract
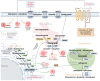
A significant challenge in cancer treatment is the rising problem of drug resistance that reduces the effectiveness of therapeutic strategies. Current knowledge shows that multiple mechanisms play a role in cancer drug resistance. Another mechanism that has gained attention is the alteration in sphingolipid trafficking and the dysregulation of its metabolism, which was reported to cause cancer-associated drug resistance. Sphingolipids are lipids containing sphingosine and have multiple roles, ranging from lipid raft formation, apoptosis, and cell signaling to immune cell trafficking. Recent studies show that in developing cancer cells, altered or dysregulated sphingolipids are associated with drug efflux and promote the survival of cancer cells by bypassing apoptosis. Upregulated levels of the glucosylceramide synthase (GCS), an enzyme that functions in sphingolipid metabolism, lead to the upregulated ABCB1 gene that induces drug efflux from the cancer cells. These bypass mechanisms make drugs that induce apoptosis in tumor cells ineffective. By highlighting the current findings, this review aims to provide a mechanism of drug resistance caused by the dysregulation of glucosylceramide synthase, sphingosine kinase, and acid ceramidase enzymes as possible therapeutic targets to enhance the effectiveness of the currently used chemotherapeutic agents.
Keywords: acid ceramidase; drug resistance; glucosylceramide synthase; sphingolipid metabolism; sphingomyelinase; sphingosine kinase; sphingosine-1-phosphate.
Conflict of interest statement
Author Shynggys Sergazy was employed by the company LLP “VICTUS PHARM”. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Kumar A., Singh K., Kumar K., Singh A., Tripathi A., Tiwari L. Drug Resistance in Cancer Therapy: Mechanisms, Challenges and Strategies. Asian J. Nurs. Educ. Res. 2024;14:95–100. doi: 10.52711/2349-2996.2024.00019. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

