Liraglutide Attenuates FFA-Induced Retinal Pigment Epithelium Dysfunction via AMPK Activation and Lipid Homeostasis Regulation in ARPE-19 Cells
- PMID: 40332323
- PMCID: PMC12027664
- DOI: 10.3390/ijms26083704
Liraglutide Attenuates FFA-Induced Retinal Pigment Epithelium Dysfunction via AMPK Activation and Lipid Homeostasis Regulation in ARPE-19 Cells
Abstract
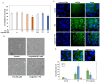
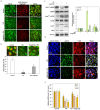
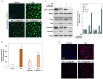
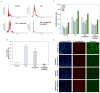
Age-related macular degeneration (AMD) is a leading cause of irreversible vision loss in the elderly, and it is characterized by oxidative stress, lipid dysregulation, and dysfunction of the retinal pigment epithelium (RPE). A hallmark of AMD is the presence of drusen, extracellular deposits rich in lipids, proteins, and cellular debris, which are secreted by the RPE. These deposits impair RPE function, promote chronic inflammation, and accelerate disease progression. Despite advancements in understanding AMD pathogenesis, therapeutic strategies targeting lipid dysregulation and oxidative damage in RPE cells remain limited. This study evaluated the effects of liraglutide, a glucagon-like peptide-1 receptor agonist (GLP-1RA), on free fatty acid (FFA)-induced damage in ARPE-19 cells, a widely used in vitro model of RPE dysfunction. FFA treatment induced lipid droplet accumulation, oxidative stress, and epithelial-mesenchymal transition (EMT), which are processes implicated in AMD progression. Liraglutide significantly reduced lipid droplet accumulation, mitigated oxidative stress, and suppressed EMT, as demonstrated by high-content imaging, immunocytochemistry, and molecular assays. Mechanistic analyses revealed that liraglutide activates AMP-activated protein kinase (AMPK), enhancing lipophagy and restoring lipid homeostasis. Furthermore, liraglutide influenced exosome secretion, altering paracrine signaling and reducing EMT markers in neighboring cells. These findings underscore liraglutide's potential to address critical mechanisms underlying AMD pathogenesis, including lipid dysregulation, oxidative stress, and EMT. This study provides foundational evidence supporting the development of GLP-1 receptor agonists as targeted therapies for AMD.
Keywords: AMP-activated protein kinase (AMPK); epithelial-mesenchymal transition (EMT); free fatty acid (FFA); lipid droplet (LD); liraglutide; retinal pigment epithelium (RPE).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Maurya M., Bora K., Blomfield A.K., Pavlovich M.C., Huang S., Liu C.H., Chen J. Oxidative stress in retinal pigment epithelium degeneration: From pathogenesis to therapeutic targets in dry age-related macular degeneration. Neural Regen. Res. 2023;18:2173–2181. doi: 10.4103/1673-5374.369098. - DOI - PMC - PubMed
-
- Wong J.H.C., Ma J.Y.W., Jobling A.I., Brandli A., Greferath U., Fletcher E.L., Vessey K.A. Exploring the pathogenesis of age-related macular degeneration: A review of the interplay between retinal pigment epithelium dysfunction and the innate immune system. Front. Neurosci. 2022;16:1009599. doi: 10.3389/fnins.2022.1009599. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- CSH-2025-C-049/Chung Shan Medical University Hospital
- 112-2320-B-040-003-MY3/National Science and Technology Council of Taiwan
- 111-2314-B-040-029-MY3/National Science and Technology Council of Taiwan
- 111-2320-B-040-017-MY3/National Science and Technology Council of Taiwan
- 113-2314-B-040-009/National Science and Technology Council of Taiwan
LinkOut - more resources
Full Text Sources
Research Materials

