A Pharmacokinetic-Pharmacodynamic Study of Protosappanoside D, a Component Derived from Biancaea decapetala Extracts, for Its Anti-Inflammatory Effects
- PMID: 40332334
- PMCID: PMC12027796
- DOI: 10.3390/ijms26083694
A Pharmacokinetic-Pharmacodynamic Study of Protosappanoside D, a Component Derived from Biancaea decapetala Extracts, for Its Anti-Inflammatory Effects
Abstract
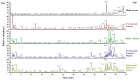
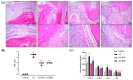
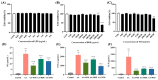
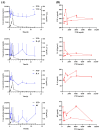
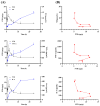
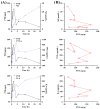
Biancaea decapetala (Roth) O. Deg. (Fabaceae), traditionally used by the Hmong people to treat rheumatoid arthritis (RA), has not been extensively studied for the correlation between its anti-inflammatory activity and its active components. Protosappanoside D (PTD), a new component, has been isolated for the first time from the extract of Biancaea decapetala. This study focused on the anti-arthritic and anti-inflammatory effects of Biancaea decapetala extracts (BDE) and PTD, along with their pharmacokinetic-pharmacodynamic (PK-PD) analysis. In the adjuvant-induced arthritis (AA) rat model, HE staining and cytokine assays showed that BDE alleviated joint damage and reduced inflammatory cytokines, similar to the positive control. In the LPS-induced inflammatory cell model, both BDE and PTD demonstrated anti-inflammatory effects by inhibiting the secretion of inflammatory factors. A PK-PD analysis of BDE in AA rats and inflammatory cells, as well as an analysis of PTD as a monomer, was conducted. The results indicated that PTD had different regulatory effects on cytokines like TNF-α, with a certain lag and sustained effects. These findings suggest the potential of BDE and PTD as treatments for rheumatoid arthritis, though further in vivo studies and clinical trials are needed.
Keywords: Biancaea decapetala; Protosappanoside D; pharmacokinetics–pharmacodynamics model; rheumatoid arthritis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Quantitative analysis of three bioactive components of Biancaea decapetala extracts in rat plasma and RAW264.7 cells using UPLC-MS/MS and its application to comparative pharmacokinetics in normal and diseased states.J Chromatogr B Analyt Technol Biomed Life Sci. 2024 Nov 1;1248:124356. doi: 10.1016/j.jchromb.2024.124356. Epub 2024 Oct 30. J Chromatogr B Analyt Technol Biomed Life Sci. 2024. PMID: 39509965
-
Biancaea decapetala (Roth) O.Deg. extract exerts an anti-inflammatory effect by regulating the TNF/Akt/NF-κB pathway.Phytomedicine. 2023 Oct;119:154983. doi: 10.1016/j.phymed.2023.154983. Epub 2023 Jul 17. Phytomedicine. 2023. PMID: 37586161
-
Identification of Protosappanoside D from Caesalpinia decapetala and Evaluation of Its Pharmacokinetic, Metabolism and Pharmacological Activity.Molecules. 2022 Sep 18;27(18):6090. doi: 10.3390/molecules27186090. Molecules. 2022. PMID: 36144821 Free PMC article.
-
Triterpene saponins from Guo-gang-long attenuate collagen-induced arthritis via regulating A20 and inhibiting MAPK pathway.J Ethnopharmacol. 2021 Apr 6;269:113707. doi: 10.1016/j.jep.2020.113707. Epub 2021 Jan 7. J Ethnopharmacol. 2021. PMID: 33358855
-
Evaluation of rohitukine-enriched fraction of Dysoxylum binectariferum Hook.f. (leaves) as anti-arthritic phytopharmaceutical candidate: Chemical standardization, in-vivo validation, formulation development and oral pharmacokinetics.J Ethnopharmacol. 2020 May 23;254:112758. doi: 10.1016/j.jep.2020.112758. Epub 2020 Mar 9. J Ethnopharmacol. 2020. PMID: 32165175
References
-
- Wang F., Song Y., Bai J., Cui D., Cheng L., Zhao J., Gao K., Shang G., Zhou X., Jia Y., et al. Application of pharmacokinetic-pharmacodynamic modeling. Chin. J. Clin. Pharmacol. 2018;34:2667–2670.
-
- Mukkavilli R., Yang C., Tanwar R.S., Saxena R., Gundala S.R., Zhang Y., Ghareeb A., Floyd S.D., Vangala S., Kuo W.W., et al. Pharmacokinetic-pharmacodynamic correlations in the development of ginger extract as an anticancer agent. Sci. Rep. 2018;8:3056. doi: 10.1038/s41598-018-21125-2. - DOI - PMC - PubMed
-
- Wang X., Liu X., Han Y., Wang Y., Zhou S., Cai B., Zhou D. Overview on main research method of effective material basis of Chinese materia medica. Chin. Trad. Herb. Drugs. 2018;49:941–947.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

