Aging-Associated Amyloid-β Plaques and Neuroinflammation in Bottlenose Dolphins (Tursiops truncatus) and Novel Cognitive Health-Supporting Roles of Pentadecanoic Acid (C15:0)
- PMID: 40332352
- PMCID: PMC12027839
- DOI: 10.3390/ijms26083746
Aging-Associated Amyloid-β Plaques and Neuroinflammation in Bottlenose Dolphins (Tursiops truncatus) and Novel Cognitive Health-Supporting Roles of Pentadecanoic Acid (C15:0)
Abstract
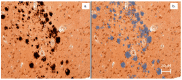
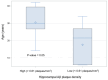
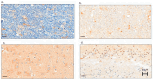
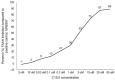
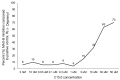
There is an urgent need to identify interventions that broadly target aging-related cognitive decline and progression to Alzheimer's disease (AD). Bottlenose dolphins (Tursiops truncatus) have histologic changes similar to AD in humans, and they also develop shared age-associated co-morbidities identified as risk factors for AD in humans, including type 2 diabetes, ferroptosis, and iron overload, which can be driven by nutritional C15:0 deficiency. We hypothesized that (1) dolphins would have amyloid beta (Aβ) plaques and neuroinflammation that paralleled that of humans in relation to age-related progression, quantitative concentration, and brain region; and (2) C15:0 would have dose-dependent activities relevant to protecting cognitive health. Quantitative immunohistochemistry staining was used to assess 68 tissues from archived brains of 19 Navy dolphins to evaluate associations among amyloid beta (Aβ) plaques and neuroinflammation by brain region, sex, and age group. Further, dose-dependent C15:0 activities, using a third-party panel intended to screen for potential AD therapeutics, were evaluated. Similar to humans, dolphins had the highest Aβ plaque density variation in the hippocampus (90th percentile of 4.95 plaques/mm2), where plaque density increased with age (p = 0.05). All measured markers of neuroinflammation were detected, including the highest concentrations of activated microglia (CD68+) in the hippocampus (0.46 ± 0.38 cells/mm2). C15:0 was a dose-dependent inhibitor of two targets, fatty acid amide hydrolase (FAAH) (IC50 2.5 µM, 89% maximum inhibition at 50 µM relative to URB597) and monoamine oxidase B (MAO-B) (IC50 19.4 µM, 70% maximum inhibition at 50 µM relative to R(-)-Deprenyl). These activities have demonstrated efficacy against Aβ formation and neuroinflammation, including protection of cognitive function in the hippocampus. These findings suggest that, in addition to protecting against AD co-morbidities, C15:0 may play a distinct role in supporting cognitive health, especially at higher concentrations.
Keywords: Alzheimer’s disease; C15:0; amyloid beta plaques; cognitive health; dolphins; fatty acid amide hydrolase (FAAH) inhibitor; monoamine oxidase B (MAO-B) inhibitor; neuroinflammation; neuroprotectant; pentadecanoic acid.
Conflict of interest statement
S.V. is a co-founder of and employed by Epitracker, Inc. and Seraphina Therapeutics, Inc., which hold exclusive licensing rights from the U.S. Navy to commercialize odd-chain saturated fatty acids as human and animal health products. The funder of this study, the Office of Naval Research, had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
References
-
- World Health Organization; Geneva, Switzerland: [(accessed on 20 January 2025)]. A Blueprint for Dementia Research. Available online: https://www.who.int/publications/i/item/9789240058248.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

