Conditional Overexpression of Neuritin in Supporting Cell Protects Cochlear Hair Cell and Delays Age-Related Hearing Loss by Enhancing Autophagy
- PMID: 40332354
- PMCID: PMC12027747
- DOI: 10.3390/ijms26083709
Conditional Overexpression of Neuritin in Supporting Cell Protects Cochlear Hair Cell and Delays Age-Related Hearing Loss by Enhancing Autophagy
Abstract
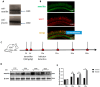
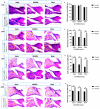
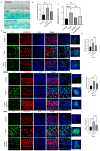
Age-related hearing loss (ARHL) is a highly prevalent, burdensome sensorineural hearing loss closely associated with impaired autophagic influx. Our previous studies revealed that neuritin, a neurotrophic factor primarily expressed in the central nervous system, could alleviate drug-induced damages in hair cells (HCs) and spiral ganglion neurons. However, its effects on ARHL and whether these effects are closely related to autophagy remain unclear. Using the Nrn1 knock-in mice and cultured cochlear basilar membrane (CBM) of the neonatal mouse, we show that neuritin could restore aging-associated hearing loss and alleviate senescence-associated damage in the cochlea. Overexpression of neuritin in support cells (SCs) alleviates the loss of cochlear HCs and nerve fibers, reducing the damage to spiral ganglion neurons and the shifts in ABR's high-frequency threshold. Furthermore, conditional overexpression of neuritin in SCs improves autophagic influx by upregulating the expression of microtubule-associated protein 1 light chain 3 type B (LCB3) protein and downregulating the expression of p21 protein. In cultured neonatal mouse CBM, neuritin administration significantly inhibits D-galactose-induced HC loss, cellular apoptosis, and ROS production and promotes autophagic influx. These effects were weakened when the autophagy inhibitor 3-MA was added. In summary, our results confirm the therapeutic potential of neuritin treatment for ARHL.
Keywords: LCB3; P21; age-related hearing loss; autophagy; hair cells; neuritin.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures







Similar articles
-
Conditional overexpression of neuritin in supporting cells (SCs) mitigates hair cell (HC) damage and induces HC regeneration in the adult mouse cochlea after drug-induced ototoxicity.Hear Res. 2022 Jul;420:108515. doi: 10.1016/j.heares.2022.108515. Epub 2022 May 11. Hear Res. 2022. PMID: 35584572
-
Codeficiency of Lysosomal Mucolipins 3 and 1 in Cochlear Hair Cells Diminishes Outer Hair Cell Longevity and Accelerates Age-Related Hearing Loss.J Neurosci. 2018 Mar 28;38(13):3177-3189. doi: 10.1523/JNEUROSCI.3368-17.2018. Epub 2018 Feb 16. J Neurosci. 2018. PMID: 29453205 Free PMC article.
-
Exogenous neuritin restores auditory following cochlear spiral ganglion neuron denervation of gerbils.Neurosci Res. 2024 Mar;200:8-19. doi: 10.1016/j.neures.2023.11.001. Epub 2023 Nov 4. Neurosci Res. 2024. PMID: 37926219
-
Pathology and mechanisms of cochlear aging.J Neurosci Res. 2020 Sep;98(9):1674-1684. doi: 10.1002/jnr.24439. Epub 2019 May 7. J Neurosci Res. 2020. PMID: 31066107 Free PMC article. Review.
-
Molecular Mechanisms and Biological Functions of Autophagy for Genetics of Hearing Impairment.Genes (Basel). 2020 Nov 11;11(11):1331. doi: 10.3390/genes11111331. Genes (Basel). 2020. PMID: 33187328 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

