Single-Cell Transcriptomic Analysis of the Potential Mechanisms of Follicular Development in Stra8-Deficient Mice
- PMID: 40332359
- PMCID: PMC12027774
- DOI: 10.3390/ijms26083734
Single-Cell Transcriptomic Analysis of the Potential Mechanisms of Follicular Development in Stra8-Deficient Mice
Abstract
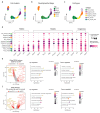
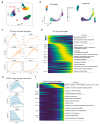
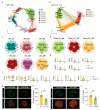
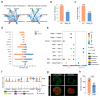
Follicle development is a critical process in mammalian reproduction, with significant implications for ovarian reserve and fertility. Stra8 is a known key factor regulating the initiation of meiosis; however, oocyte-like cells still appear in Stra8-deficient mice. Nevertheless, the underlying mechanism remains unclear and requires further investigation. Therefore, we used single-cell RNA sequencing to construct a comprehensive transcriptional atlas of ovarian cells from both wild-type and Stra8-deficient mice at embryonic stages E14.5 and E16.5. With stringent quality control, we obtained a total of 14,755 single cells of six major cell types. A further fine-scale analysis of the germ cell clusters revealed notable heterogeneity between wild-type and Stra8-deficient mice. Compared to the wild-type mice, the deficiency in Stra8 led to the downregulation of meiosis-related genes (e.g., Pigp, Tex12, and Sycp3), and the upregulation of apoptosis-related genes (e.g., Fos, Jun, and Actb), thereby hindering the meiotic process. Notably, we observed that, following Stra8 deficiency, the expression levels of Sub1 and Stk31 remained elevated at this stage. Furthermore, an RNA interference analysis confirmed the potential role of these genes as regulatory factors in the formation of primordial follicle-like cells. Additionally, Stra8 deficiency disrupted the signaling between germ cells and pregranulosa cells that is mediated by Mdk-Sdc1, leading to the abnormal expression of the PI3K/AKT signaling pathway. Together, these results shed light on the molecular processes governing germ cell differentiation and folliculogenesis, emphasizing the complex role of Stra8 in ovarian function.
Keywords: Stra8; oogenesis; single-cell RNA sequencing.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
BMP and STRA8 act collaboratively to ensure correct mitotic-to-meiotic transition in the fetal mouse ovary.Development. 2025 Feb 1;152(3):DEV204227. doi: 10.1242/dev.204227. Epub 2025 Feb 7. Development. 2025. PMID: 39817676 Free PMC article.
-
Meiotic gatekeeper STRA8 suppresses autophagy by repressing Nr1d1 expression during spermatogenesis in mice.PLoS Genet. 2019 May 6;15(5):e1008084. doi: 10.1371/journal.pgen.1008084. eCollection 2019 May. PLoS Genet. 2019. PMID: 31059511 Free PMC article.
-
Oocyte differentiation is genetically dissociable from meiosis in mice.Nat Genet. 2013 Aug;45(8):877-83. doi: 10.1038/ng.2672. Epub 2013 Jun 16. Nat Genet. 2013. PMID: 23770609 Free PMC article.
-
Mechanism of initiation of meiosis in mouse germ cells.Curr Top Dev Biol. 2023;151:1-26. doi: 10.1016/bs.ctdb.2022.04.005. Epub 2022 Jun 3. Curr Top Dev Biol. 2023. PMID: 36681467 Review.
-
Female-specific mechanisms of meiotic initiation and progression in mammalian oocyte development.Genes Cells. 2024 Oct;29(10):797-807. doi: 10.1111/gtc.13152. Epub 2024 Aug 9. Genes Cells. 2024. PMID: 39119753 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
- 32100683/National Nature Science Foundation of China
- ZR2021QC003/the Natural Science Foundation of Shandong Province
- ts20190946 and tsqn202211194/the Taishan Scholar Construction Foundation of Shandong Province of China
- 6651121003/the Start-up Fund for High-level Talents of Qingdao Agricultural University
LinkOut - more resources
Full Text Sources
Miscellaneous

