Lapatinib-Resistant HER2+ Breast Cancer Cells Are Associated with Dysregulation of MAPK and p70S6K/PDCD4 Pathways and Calcium Management, Influence of Cryptotanshinone
- PMID: 40332401
- PMCID: PMC12027730
- DOI: 10.3390/ijms26083763
Lapatinib-Resistant HER2+ Breast Cancer Cells Are Associated with Dysregulation of MAPK and p70S6K/PDCD4 Pathways and Calcium Management, Influence of Cryptotanshinone
Abstract
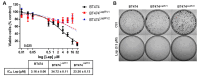
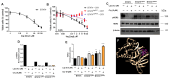
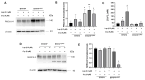
Resistance to HER2 tyrosine-kinase inhibitor Lapatinib (Lap) is one of the leading causes of cancer treatment failure in HER2+ breast cancer (BC), associated with an aggressive tumor phenotype. Cryptotanshinone (Cry) is a natural terpene molecule that could function as a chemosensitizer by disturbing estrogen receptor (ERα) signaling and inhibiting the protein translation factor-4A, eIF4A. Therefore, we evaluated Cry dual regulation on eIF4A and ERα. This study aimed to elucidate the underlying mechanisms of Lap chemoresistance and the impact of Cry on them. We generated two Lap-resistant BT474 cell HER2+ variants named BT474LapRV1 and BT474LapRV2 with high chemoresistance levels, with 7- and 11-fold increases in EC50, respectively, compared to BT474 parental cells. We found a PDCD4-p70S6Kβ axis association with Lap chemoresistance. However, a concomitant down-regulation of the RAF-MEK-ERK cell survival pathway and NF-κB was found in the chemoresistant cell variants; this phenomenon was exacerbated by joint treatment of Cry and Lap under a Lap plasmatic reported concentration. Optimized calcium management was identified as a compensatory mechanism contributing to chemoresistance, as determined by the higher expression of calcium pumps PMCA1/4 and SERCA2. Contrary to expectations, a combination of Lap and Cry did not affect the chemoresistance despite the ERα down-regulation; Cry-eIF4A binding possibly dampens this condition. Results indicated the pro-survival eIF4A/STAT/Bcl-xl pathway and that the down-regulation of the MAPK-NF-κB might function as an adaptive mechanism; this response may be compensated by calcium homeostasis in chemoresistance, highlighting new adaptations in HER2+ cells that lead to chemoresistance.
Keywords: breast cancer HER2+; calcium homeostasis; chemoresistance; cryptotanshinone; lapatinib.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





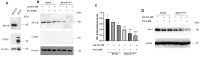
References
MeSH terms
Substances
Grants and funding
- A1-S-28653/SEP/CONACYT/Fondo Sectorial de Investigación para la Educación CB 2017-2018
- Programas Nacionales Estratégicos de Ciencia, Tecnología y Vinculación con los Sectores Social, Público y Privado/Programa Presupuestario F003
- 23a. Convocatoria Interna de Apoyo a Proyectos de Investigación (2-12-213-2-003-2-1994)/Coordinación General de Investigación y Posgrado/UABC
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

