Co-Encapsulation of Multiple Antineoplastic Agents in Liposomes by Exploring Microfluidics
- PMID: 40332493
- PMCID: PMC12027889
- DOI: 10.3390/ijms26083820
Co-Encapsulation of Multiple Antineoplastic Agents in Liposomes by Exploring Microfluidics
Abstract
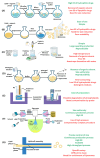
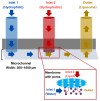
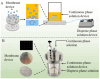
The inherent complexity of cancer proliferation and malignancy cannot be addressed by the conventional approach of relying on high doses of a single powerful anticancer agent, which is associated with poor efficacy, higher toxicity, and the development of drug resistance. Multiple drug therapy (MDT) rationally designed to target tumor heterogeneity, block alternative survival pathways, modulate the tumor microenvironment, and reduce toxicities would be a viable solution against cancer. Liposomes are the most suitable carrier for anticancer MDT due to their ability to encapsulate both hydrophilic and hydrophobic agents, biocompatibility, and controlled release properties; however, an adequate manufacturing method is important for effective co-encapsulation. Microfluidics involves the manipulation of fluids at the microscale for the controlled synthesis of liposomes with desirable properties. This work critically reviews the use of microfluidics for the synthesis of anticancer MDT liposomes. MDT success not only relies on the identification of synergistic dose combinations of the anticancer modalities but also warrants the loading of multiple therapeutic entities within liposomes in optimal ratios, the protection of the drugs by the nanocarrier during systemic circulation, and the synchronous release at the target site in the same pattern as confirmed in preliminary efficacy studies. Prospects have been identified for the bench-to-bedside translation of anticancer MDT liposomes using microfluidics.
Keywords: cancer; drug delivery; liposomes; microfluidics; multiple drug therapy.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Kadam U.T., Roberts I., White S., Bednall R., Khunti K., Nilsson P.M., Lawson C.A. Conceptualizing multiple drug use in patients with comorbidity and multimorbidity: Proposal for standard definitions beyond the term polypharmacy. J. Clin. Epidemiol. 2019;106:98–107. doi: 10.1016/j.jclinepi.2018.10.014. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

