Targeting the Ischemic Core: A Therapeutic Microdialytic Approach to Prevent Neuronal Death and Restore Functional Behaviors
- PMID: 40332503
- PMCID: PMC12027531
- DOI: 10.3390/ijms26083821
Targeting the Ischemic Core: A Therapeutic Microdialytic Approach to Prevent Neuronal Death and Restore Functional Behaviors
Abstract
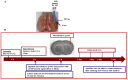
Ischemic stroke leads to cerebral ionic imbalance, increases acidosis, oxidative stress and release of glutamate and inflammatory mediators. Removing solute or stimulants from the ischemic core may block cell-damaging events and confer neuroprotection. In this study, we developed a minimally invasive therapeutic microdialysis (tMD) method, choosing to include serum albumin in the buffer because it is a multifunctional protein with osmotic properties. Aiming at the ischemic core, continuous perfusion of buffer supplemented with osmotic agents removes mediators of inflammation/cell damage/death from the lesion. This tMD treatment significantly removed the glutamate and zinc ions from the core, thereby reducing infarct volumes and affording high-grade neurobehavioral protection against ischemic stroke. The tMD treatment effectively protected neurons and reduced microglial activation. Furthermore, this tMD approach extended the therapeutic window to protect beyond 6 h after stroke onset. These findings support the potential clinical feasibility of applying tMD to patients with ischemic stroke, potentially without adverse effects.
Keywords: cerebral ischemia; microdialysis; neuroprotection; serum albumin.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Fecal microbiota transplantation alleviates neuronal Apoptosis, necroptosis and reactive microglia activation after ischemic stroke.Neuroscience. 2025 Jan 9;564:299-305. doi: 10.1016/j.neuroscience.2024.10.053. Epub 2024 Nov 2. Neuroscience. 2025. PMID: 39491609
-
Stress primes microglial polarization after global ischemia: Therapeutic potential of progesterone.Brain Behav Immun. 2017 Nov;66:177-192. doi: 10.1016/j.bbi.2017.06.012. Epub 2017 Jun 23. Brain Behav Immun. 2017. PMID: 28648389
-
Fluoxetine and citalopram decrease microglial release of glutamate and D-serine to promote cortical neuronal viability following ischemic insult.Mol Cell Neurosci. 2013 Sep;56:365-74. doi: 10.1016/j.mcn.2013.07.006. Epub 2013 Jul 19. Mol Cell Neurosci. 2013. PMID: 23876875
-
Neuronal injuries in cerebral infarction and ischemic stroke: From mechanisms to treatment (Review).Int J Mol Med. 2022 Feb;49(2):15. doi: 10.3892/ijmm.2021.5070. Epub 2021 Dec 8. Int J Mol Med. 2022. PMID: 34878154 Free PMC article. Review.
-
Exploring the intricacies of calcium dysregulation in ischemic stroke: Insights into neuronal cell death and therapeutic strategies.Life Sci. 2024 Jun 15;347:122651. doi: 10.1016/j.lfs.2024.122651. Epub 2024 Apr 19. Life Sci. 2024. PMID: 38642844 Review.
References
-
- Feigin V.L., Owolabi M.O., World Stroke Organization-Lancet Neurology Commission Stroke Collaboration Group Pragmatic solutions to reduce the global burden of stroke: A World Stroke Organization-Lancet Neurology Commission. Lancet Neurol. 2023;22:1160–1206. doi: 10.1016/S1474-4422(23)00277-6. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

