Epigenetic Inactivation of RIPK3-Dependent Necroptosis Augments Cisplatin Chemoresistance in Human Osteosarcoma
- PMID: 40332549
- PMCID: PMC12027565
- DOI: 10.3390/ijms26083863
Epigenetic Inactivation of RIPK3-Dependent Necroptosis Augments Cisplatin Chemoresistance in Human Osteosarcoma
Abstract
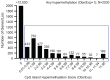
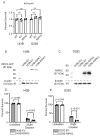
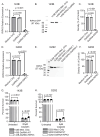
Osteosarcoma (OS) is the most common primary bone malignancy in children and adolescents. Unfortunately, drug resistance limits the efficacy of chemotherapeutic treatment and compromises therapeutic outcomes in a substantial proportion of cases. Aberrant CpG island methylation-associated transcriptional silencing contributes to chemoresistance in pediatric solid tumors. Here, using whole-genome DNA methylation screening on 16 human primary OS specimens, we identify receptor interacting protein kinase-3 (RIPK3), a molecular regulator of the necroptosis programmed cell death pathway, as a gene target of aberrant CpG methylation and demonstrate its role in human OS chemoresistance. We validated these findings via enforced expression and DsiRNA silencing, and evaluated the role of RIPK3 in cisplatin chemosensitivity and necroptosis activation through MLKL phosphorylation. We found that CpG island methylation results in RIPK3 silencing in primary human OS samples and cell lines. Enforced RIPK3 expression significantly enhanced cisplatin cytotoxicity in OS cells and DsiRNA knockdown reversed the cisplatin-sensitive phenotype. In cells with enforced RIPK3 expression, cisplatin treatment significantly increased phosphorylation of both RIPK3 and its target, MLKL, indicative of induction of necroptosis. Here, we identify RIPK3 as an important mediator of chemoresistance in OS and a potential pharmacologic target to improve chemotherapy efficacy in drug-resistant tumors.
Keywords: CpG islands; RIPK3; chemoresistance; necroptosis; osteosarcoma.
Conflict of interest statement
All authors declare no conflicts of interest.
Figures


Similar articles
-
Somatic Epigenetic Silencing of RIPK3 Inactivates Necroptosis and Contributes to Chemoresistance in Malignant Mesothelioma.Clin Cancer Res. 2021 Feb 15;27(4):1200-1213. doi: 10.1158/1078-0432.CCR-18-3683. Epub 2020 Nov 17. Clin Cancer Res. 2021. PMID: 33203643 Free PMC article.
-
TGM2 knockdown reverses cisplatin chemoresistance in osteosarcoma.Int J Mol Med. 2018 Oct;42(4):1799-1808. doi: 10.3892/ijmm.2018.3753. Epub 2018 Jul 4. Int J Mol Med. 2018. PMID: 30015899 Free PMC article.
-
CCN2 enhances resistance to cisplatin-mediating cell apoptosis in human osteosarcoma.PLoS One. 2014 Mar 17;9(3):e90159. doi: 10.1371/journal.pone.0090159. eCollection 2014. PLoS One. 2014. PMID: 24637722 Free PMC article.
-
Long Non-coding RNAs in Cisplatin Resistance in Osteosarcoma.Curr Treat Options Oncol. 2021 Mar 20;22(5):41. doi: 10.1007/s11864-021-00839-y. Curr Treat Options Oncol. 2021. PMID: 33745006 Review.
-
MicroRNAs as the pivotal regulators of cisplatin resistance in osteosarcoma.Pathol Res Pract. 2023 Sep;249:154743. doi: 10.1016/j.prp.2023.154743. Epub 2023 Aug 6. Pathol Res Pract. 2023. PMID: 37549518 Review.
References
-
- Kuerbitz S.J., Henderson M.B. Osteosarcoma: A review with emphasis on pathogenesis and chemoresistance. Med. Res. Arch. 2020;8:1–37. doi: 10.18103/mra.v8i7.2170. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

